Frailty domain
Method of measurement
Cutoffs for measurement
1. Slowness
5-m gait speed
Patient is asked to walk at a comfortable pace from a 0–m start line to past a 5–m finish line, the cue to start and stop the stopwatch is the first footfall after the start line and first footfall after the finish line, this is repeated 3 times and the average time is recorded
Sex- and height-based cutoff
♂
≤173 cm
≤0.65 m/s
>173 cm
≤0.76 m/s
♀
≤159 cm
≤0.65 m/s
>159 cm
≤0.76 m/s
Simplified alternative cutoff
♂/♀: ≤0.83 m/s
2. Weakness
Handgrip strength
Patient is asked to squeeze a handgrip dynamometer as hard as possible, this is repeated 3 times (with each hand and then with the strongest hand) and the maximum value is recorded
Sex- and BSA-based cutoff
♂
≤24 kg/m2
≤29 kg
24.1–28 kg/m2
≤30 kg
>28 kg/m2
≤32 kg
♀
≤26 kg/m2
≤17 kg
26.1–29 kg/m2
≤18 kg
>29 kg/m2
≤21 kg
Simplified alternative cutoff
♂
≤30 kg
♀
≤20 kg
3. Low physical activity
Minnesota Leisure-time physical activity questionnaire103
♂
<383 kcal/week
♀
<270 kcal/week
4. Weight loss
Self-reported
>10 lbs or >5 % over the past year
5. Exhaustion
2 questions: How often do you feel
“Everything I did was an effort”
“I could not get going”
Positive if answered either: “Most of the time” or “Moderate amount of the time”
≥3 criteria required for a diagnosis of frailty
At the physiological level, frailty is typified by impaired homeostatic reserve and reduced resiliency to stressors either related to illness or iatrogenic factors. When faced with exacerbations or invasive therapies, as is commonly encountered towards the EOL, frail patients tend to demonstrate marked and often disproportionate decompensation in health status and functional capacity. At the epidemiological level, frailty is strongly associated with risk of cardiac and all-cause mortality, complications after cardiovascular interventions, institutionalization, and lower quality of life [9, 10].
Disability is an inter-related but distinct concept from frailty, that is defined as inability or dependency to carry out basic and instrumental activities of daily living (ADL and IADL, respectively) [11]. The Katz [12], Barthel [13], and Older Americans Resources and Services (OARS) [14] scales are used in research and in practice to assess disabilities. Disabilities surface in the later stages of the frailty spectrum, once patients have faced significant stressors and accrued deficits. Importantly, disabilities in ADLs have been shown to be harbingers of EOL, signifying an expected survival less than 6 months in many independent studies [15, 16].
Cognitive Impairment
CI is defined as impairment in one or more cognitive function domains, namely: memory and learning, language, executive function, complex attention, perceptual-motor, and social cognition. Following the criteria set forth by the Diagnostic and Statistical Manual (DSM), these impairments must be acquired and represent a significant decline from baseline, they must interfere with independence in everyday activities, and not occur exclusively during the course of delirium. The diagnosis is insidious, and often missed or overlooked in practice. We and others have observed that dementia or mild CI is documented in admission notes and discharge summaries in 5–10 % of older cardiology ward patients, whereas it is elicited by cognitive testing in over one-third of such patients [17]. When CI is present but not documented or recognized by the treating physicians, the risk of mortality and hospital readmission increases significantly [2]. Mild CI may be falsely attributed to “normal aging” and even significant dementia may be attributed to behavioral or personality issues. This underscores the importance of cognitive testing rather than self-report or subjective assessment to establish the presence or absence of CI.
A number of cognitive testing instruments have been developed and validated. The Mini-Mental Status Examination (MMSE) [18] spans orientation, registration, attention and calculation, recall, and language. A score ≥27/30 is normal, 21–26 is mild impairment, 11–20 is moderate impairment, and ≤10 is severe impairment (another classification scheme states that ≥24 is normal, 18–23 is mild-moderate impairment, and ≤17 is severe impairment); cutoffs exist adjusted for age, sex, and educational level. The limitations of the MMSE are that it is less sensitive to mild CI, and it is proprietary. The Montreal Cognitive Assessment (MoCA) [19] – available at www.mocatest.org – overcomes these limitations, and furthermore, was found to be more predictive of adverse outcomes in heart failure patients [20, 21]. MoCA spans visuospatial and executive function, naming, memory, attention, language, abstraction, delayed recall, and orientation. A score <26 is abnormal; severity is determined by the burden of functional impairments. Brief instruments such as the Mini-Cog employ a clock drawing test and 3-word recall to efficiently screen for dementia (positive if 0 words recalled, or 1–2 words recalled plus abnormal clock draw).
Dementia may be sub-classified as neurodegenerative or non-neurodegenerative, with the former consisting of these major dementia syndromes: Alzheimer disease, vascular dementia, Lewy body disease, frontotemporal dementia, and Parkinson disease. Non-neurodegenerative etiologies must be carefully considered in patients with advanced CVD, since these tend to manifest in a subclinical yet nefarious fashion, consisting of medication side effects, metabolic disturbances, depression, and other differential diagnoses reviewed in section V.
Frailty and Cognitive Impairment as Cues to Consider Palliative Care
Frailty and CI Can Refine Predictions of Expected Survival
One of the primordial challenges in delivering effective EOL care is knowing when EOL is near in order to engage the patient in shared EOL decision making and activate palliative care (PC) resources in a timely manner. Patient and family satisfaction is increased when PC is initiated earlier in the disease trajectory. Prediction of expected survival is used by clinicians to guide this transition, which is gradual rather than abrupt, from curative to palliative therapies. Unfortunately, only 15 % of clinicians surveyed believed that they could reliably predict EOL in their heart failure patients [22], and risk models to predict expected survival in non-cancer conditions are inaccurate in the last 6 months of life. A systematic review of 11 risk models concluded that “no prognostic model can be recommended” [23]. Heart failure-specific risk models were not developed to predict 6-month survival, rather focusing on in-hospital (<30 days; ADHERE [24] and EFFECT [25] risk models) or long-term (>1–5 years; Seattle Heart Failure Model [26]) survival.
To address this, Huynh and Rich developed a risk model to predict 6-month survival tailored to older patients with heart failure who may be candidates for hospice care [27]. The following seven items emerged in multivariable analysis, with ≥4/7 items signifying a high risk of death at 6 months: age ≥75, dementia, coronary artery disease, peripheral arterial disease, systolic blood pressure <120 mmHg, BUN ≥30 mg/dL, Na <135 mEq/L. Dementia was among the most powerful predictors, with an adjusted hazard ratio of 2.02. In another study, the combination of MMSE score <18 plus Barthel ADL score <90 had a synergistic effect on predicting 6-month survival [28].
Among the risk models that predict 30-day survival, Pilotto and Ferrucci compared their Multidimensional Prognostic Index (MPI) risk score based on a comprehensive geriatric assessment including CI and ADL/IADL disabilities (Table 14.2 and Fig. 14.1) [29] to the ADHERE and EFFECT risk scores that are based solely on cardiac parameters and do not encompass geriatric parameters. The MPI substantially outperformed other risk scores (C-statistic 0.80–0.83 vs. 0.65–0.71) indicating the value of considering geriatric domains to predict EOL in vulnerable heart failure patients.
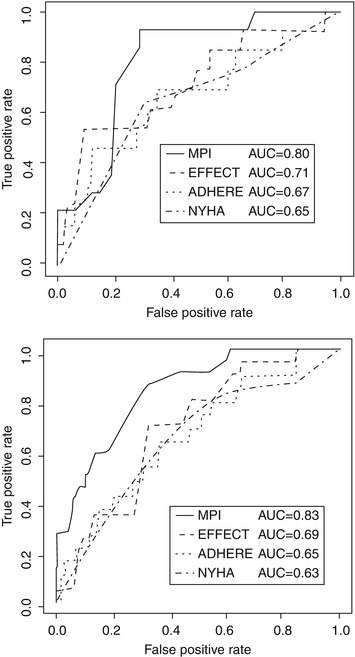
Table 14.2
Multidimensional prognostic index
Assessment | Problems | ||
|---|---|---|---|
No | Minor | Severe | |
(Value = 0) | (Value = 0.5) | (Value = 1) | |
ADLa | 6–5 | 4–3 | 2–0 |
Instrumental ADLa | 8–6 | 5–4 | 3–0 |
Short portable mental status questionnaireb | 0–3 | 4–7 | 8–10 |
Comorbidity indexc | 0 | 1–2 | ≥3 |
Mini nutritional assessmentd | ≥24 | 17–23.5 | <17 |
Exton-smith scalee | 16–20 | 10–15 | 5–9 |
No. of medications | 0–3 | 4–6 | ≥7 |
Social support network | Living with family | Institutionalized | Living alone |

Fig. 14.1
ROC curves for the MPI, NYHA, EFFECT, and ADHERE risk scores at 30 days of follow-up in men (bottom) and women (top) (From Pilotto et al. [29]. Reprinted with permission from Wolters Kluwer Health)
Strictly speaking, frailty is not included in the aforementioned risk models, although there is evidence to suggest that frailty is strongly associated with 6-month mortality incremental to traditional risk models [30]. Frail older adults hospitalized with severe CAD had a two- to four-fold increase in 6-month mortality [31], lower QOL, lower physical and mental functioning scores [32] compared to their nonfrail counterparts. Similar data have been reported in advanced heart failure and valvular heart disease. Recurrent hospitalizations should alert the clinician that their patient is likely to be frail, and also likely to be approaching EOL.
Thus, it is abundantly clear that CI and frailty are powerful predictors of survival in CVD, and that integrating CI and frailty within the process of risk prediction refines estimates of survival, and allows clinicians to ascertain with greater confidence when EOL may be approaching in their patients.
Identify Patients That Benefit Most from Palliative Care
According to the revised framework, the indications for PC should be driven not by prognosis, but by the needs of the patients [33] (in comparison to hospice care, which is driven by expected survival <6 months). This extends beyond medical needs, to psychological and spiritual needs, as well as social and family-related needs. Such needs are preeminent in frail and CI patients. Frail patients have an increased frequency of depression, anxiety, and social isolation. CI patients have an increased frequency of untreated symptoms, poor self-care, and family stress [34]. The impact on family members may be severe, with a high incidence of depression and prolonged bereavement noted in those who were taking care of their relative with dementia [35]. These issues are optimally addressed and managed by a specialist in PC.
When comparing two EOL cohorts, one with end-stage heart failure and one with cancer, heart failure patients were less likely to receive a prescription for opiates (22 % vs. 46 %) and less likely to be referred for hospice care (20 % vs. 51 %) [36]. Heart failure patients were more likely to be admitted for acute care services in the 30 days preceding death (60 % vs. 45 %) and to be admitted to the intensive care unit for aggressive management (19 % vs. 7 %). CI was an independent predictor for not receiving an opiate prescription. Interestingly, another study that compared end-stage heart failure to cancer found that the needs for PC were equally justifiable in heart failure given the high symptom burden, depression, and low spiritual well-being [37].
Patients following the frailty and dementia trajectories have the highest risk of developing significant dependencies in ADLs towards the EOL [38, 39]. Active involvement of geriatrics, physiotherapy, occupational therapy, and home care services should be sought to limit the burden of disability and help patients maintain an independent or semi-independent lifestyle in the EOL period.
Different Diagnosis and Management of Frailty and Cognitive Impairment at End-of-Life
Approach to Cognitive Impairment in the EOL Cardiovascular Patient
An elderly patient presenting signs and symptoms of CI should not be assumed to have a form of neurodegenerative dementia, even if Alzheimers disease and vascular dementia are exceedingly common. In the setting of advanced CVD, a number of other potentially reversible causes should be sought (Fig. 14.2).
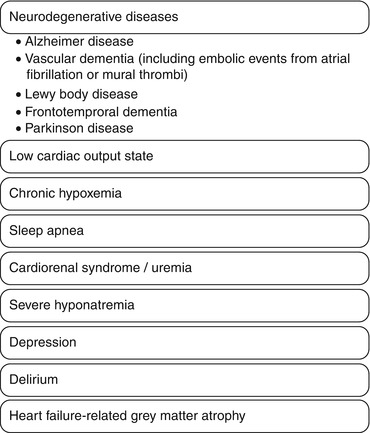

Fig. 14.2
Differential diagnosis of cognitive impairment in cardiac patients
First, in the presence of a low cardiac output, cerebral hypoperfusion has been postulated to cause CI. The evidence to support this is mixed, with studies showing that cerebral blood flow by transcranial Doppler is [40] and is not [41] associated with CI, and studies showing that both hypotension [42] and hypertension [43] are associated with CI. Anecdotally, we have observed rapid and dramatic changes in cognitive function in some heart failure patients after a brief course of intravenous vasodilators or inotropic “holiday”, although guidelines do not necessarily endorse this as routine practice. Second, embolic events due to occult atrial fibrillation or left ventricular thrombi should be excluded. Third, hypoxemia due to chronic heart failure and/or sleep apnea can cause CI, and extrapolating from the pulmonary literature, home oxygen therapy and nocturnal positive airway pressure have been shown to improve cognitive function [44, 45]. Fourth, metabolic disturbances such as uremic renal failure or severe hyponatremia may manifest as forgetfulness, confusion, and decreased attention span, and are usually improved by reducing diuretic dosage in prerenal failure or increasing diuretic dosage and restricting fluid intake in hyponatremia. Fifth, depression is one of the great masqueraders that must be excluded, as it is highly prevalent in patients with advanced CVD. Sixth, delirium should be differentiated from CI, especially in patients presenting with an acute change from baseline. Lastly, if CI is confirmed but there is no neurodegenerative disease or reversible cause identified, severe heart failure itself has been directly linked to CI by way of accelerated grey matter atrophy [46].
Approach to Frailty Signs and Symptoms in the EOL Cardiovascular Patient
Loss of muscle mass and strength are cardinal signs of frailty and also of advanced heart failure, termed sarcopenia in the former and cardiac cachexia in the latter [47]. Sarcopenia is a geriatric syndrome defined by low muscle mass and either low muscle strength or slow gait speed; its causes are multifactorial and may be equated to accelerated or unsuccessful aging of the musculature. Cachexia is a metabolic syndrome defined by weight loss and either low muscle mass, low muscle strength, fatigue, anorexia, hypoalbuminemia, anemia, or elevated inflammatory markers; it is caused by inflammatory cytokines associated with various pathological disease states. Evidently, there is room for overlap between these two syndromes, yet their pathophysiology and therapeutic options differ. Sarcopenia may respond to exercise and nutritional interventions and androgen replacement therapy [48], whereas cachexia is less often modifiable as it requires treatment of the underlying disease.
Anergia, exhaustion, and low physical activity are also cardinal symptoms of frailty with a broad differential diagnosis. Before attributing these symptoms to frailty, the clinician should exclude significant anemia, hypothyroidism, depression, sleep apnea, metabolic disturbances, iatrogenic, or worsening low cardiac output state. The presence of any of these should direct the clinician toward a targeted treatment, whereas if attributed to frailty, then the plan should revolve around a graded exercise intervention and perhaps a trial of cognitive behavioral therapy [49, 50].
Overcoming Barriers to Improve End-of-Life Care for the Frail and Cognitively Impaired
Predicting EOL
Clinicians are not equipped to predict EOL with an acceptable degree of accuracy using traditional risk models. The frailty and dementia trajectories toward death have been shown to be protracted and particularly difficult to predict. To improve risk prediction, clinicians are encouraged to integrate frailty and CI and/or utilize a model such as the MPI that encompasses geriatric domains rather than cardiac-only domains. Among frail patients, the severe form that is approaching EOL often has one or more of these features: muscle wasting, malnutrition, inability to mobilize, and disabilities in ADLs. Among CI patients, these features have been associated with <6-month survival: muscle wasting, anorexia, dehydration, pneumonia, and disabilities in ADLs [34].
Recognizing Symptoms
In advanced dementia, patients may have difficulty expressing symptoms of pain and dyspnea. Physicians and nurses express a lack of comfort and expertise to detect and quantify pain in CI patients, and benefit from continuing medical education in this regard. Moreover, instruments such as the Pain Assessment IN Advanced Dementia (PAINAD) scale (Table 14.3) [51] are available to assist in detecting pain by way of facial expressions, vocalization, and body language.
Table 14.3
Pain assessment in advanced dementia (PAINAD) scale
0 | 1 | 2 | |
|---|---|---|---|
Breathing (independent of vocalization) | Normal | Occasional labored breathing. Short period of hyperventilation | Noisy labored breathing. Long period of hyperventilation. Cheyne-stokes respirations |
Negative vocalization | None | Occasional moan or groan. Low- level of speech with a negative or disapproving quality | Repeated troubled calling out. Loud moaning or groaning. Crying |
Facial expression | Smiling or inexpressive | Sad, frightened, frown | Facial grimacing |
Body language | Relaxed | Tense. Distressed pacing. Fidgeting | Rigid. Fists clenched. Knees pulled up. Pulling or pushing away. Striking out |
Consolability | No need to console | Distracted or reassured by voice or touch | Unable to console, distract or reassure |
Managing Symptoms
Studies have shown that elderly cardiovascular patients are often either under-treated, or over-treated with burdensome interventions or polypharmacy. Before adding a new medication to manage symptoms at the EOL, it is imperative to examine the current prescriptions and consider removing non-essential medications that may be causing or aggravating symptoms. Symptom-alleviating medications such as diuretics, nitrates, and opioids may be favored over ACE inhibitors and beta-blockers. If a medication is to be added, the mantra of “start low and go slow, but get there” should be followed, meaning that low doses and slow titrations are employed while meticulously pursuing relief of symptoms [52].
In addition to pain and dyspnea, behavioral and psychological symptoms such as agitation and restlessness are common in CI patients at EOL [53]. Non-pharmacological interventions should be attempted as first line, including: reality orientation, music therapy, environment modification, and cognitive behavioral therapy. Neuroleptic medications may be required as second line, and these have been shown to be efficacious in short-term studies <6–12 weeks; however, there does not appear to be a sustained benefit >12 weeks and there is a risk of increased drowsiness and worsened quality of life [54]. Serotonin reuptake inhibitors may be used to treat depression, which is co-prevalent with frailty in many CVD patients.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


