Assessing Platelet Function in the Cath Lab
Jay S. Varanasi
Steven R. Steinhubl
Antiplatelet agents are a critical component of the adjunctive therapies utilized in patients undergoing a percutaneous coronary intervention (PCI). In fact, no other therapy—mechanical or pharmacologic—has been proven to be more effective at preventing the thrombotic complications associated with this procedure. Antiplatelet agents are most often administered as a standard dose in all patients, with the only individual patient characteristic that routinely influences the dose of an antiplatelet therapy being body weight—and this only impacts the dosing of platelet glycoprotein (GP) IIb/IIIa antagonists. This practice continues even in light of the fact that every study, in both animals and humans, which has evaluated the response to a specific thrombogenic stimulus has consistently found a wide range in interpatient variability. Similarly, all studies that have evaluated the response to antiplatelet therapies also have demonstrated a wide range in responses, with larger studies showing a normal distribution of responses, consistent with a poly-environmental and poly-genetic influence.
Monitoring the effects of antiplatelet agents currently is not routinely performed, primarily due to the lack of a convenient, proven, and clinically relevant measure of platelet function. This chapter reviews the data supporting the clinical importance of interpatient variability in response to antiplatelet agents, particularly in the setting of a PCI, and the various platelet function tests available.
ASPIRIN RESPONSIVENESS
Variability in response to the antiplatelet effect of aspirin (ASA) has been recognized for almost 40 years, and virtually every study since has found a nonuniform response to ASA among different individuals (1). However, despite the fact that this phenomenon has been recognized for so long, the clinical relevance and appropriate management of a specific ex vivo measure of ASA responsiveness remain critically important unknowns.
One of the first studies looking at clinical outcomes associated with the varying response to aspirin, by Grotemeyer et al., used the platelet aggregate ratio to measure aspirin responsiveness. Platelets disaggregate in pure ethylenediaminetetraacetate (EDTA), while they remain fixed in a mixture of formalin and EDTA. The calculated platelet aggregate ratio is the platelet count in EDTA versus the platelet count in the mixture. The ratio is <1 when platelet aggregates are present (5). In 60 of 180 stroke patients (33%), enhanced platelet reactivity was noted 12 hours after the oral administration of 500 mg of aspirin. After 2 years, the rate of stroke, MI, and vascular death was significantly higher in those patients who were apparently less responsive to aspirin (40% versus 4.4%, p <0.0001) (6).
Clinical outcomes were also evaluated in a subset of chronic aspirin users enrolled in the Heart Outcomes and Prevention Evaluation (HOPE) trial (7). An insufficient
inhibition of thromboxane production by aspirin may be represented by an increase in the urinary metabolite, 11-dehydro thromboxane B2, thus serving as an indirect measure of aspirin response. This case control analysis showed that patients suffering adverse events had significantly elevated levels of this urinary metabolite. Higher levels of 11-dehydro thromboxane B2 were associated with higher risk (8).
inhibition of thromboxane production by aspirin may be represented by an increase in the urinary metabolite, 11-dehydro thromboxane B2, thus serving as an indirect measure of aspirin response. This case control analysis showed that patients suffering adverse events had significantly elevated levels of this urinary metabolite. Higher levels of 11-dehydro thromboxane B2 were associated with higher risk (8).
Gum et al. studied 325 patients with coronary artery disease on aspirin therapy and used optical aggregation to determine aspirin responsiveness. Aspirin resistance was defined as a mean platelet aggregation of ≥70% with 10 μM adenosine diphosphate (ADP) and ≥20% with 0.5% μg/mL arachidonic acid. The study showed a continuum of aspirin responsiveness in which 5% of patients were classified as nonresponders and 23% were semiresponders (those who met one of the above criteria). These patients were followed for a mean duration of nearly 2 years. Aspirin nonresponders had a significantly higher combined rate of myocardial infarction (MI), death, and stroke compared to all others (24% versus 10%, p = 0.03) (9,10). The PFA-100 point-of-care test also was used in this study to evaluate the effects of aspirin. Unlike optical aggregometry, adverse events did not correlate to an impaired response as measured by the PFA-100.
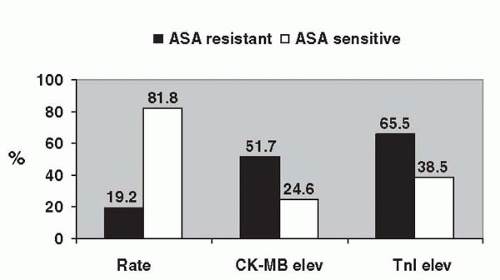
The clinical consequences of the variability in response to aspirin among patients undergoing percutaneous revascularization have been evaluated in only one study. Chen et al. studied 151 patients who were on aspirin for at least 7 days and were pretreated with clopidogrel at least 12 hours prior to elective PCI (11). Aspirin resistance, determined ex vivo using the Ultegra system, was associated with a 2.9-fold increased incidence of myonecrosis, as measured by creatine kinase (CK-MB) elevation (Fig. 50.1).
With multiple ex vivo modalities used to measure aspirin response, no standard definition of an inadequate response to aspirin has emerged. Although some have characterized patients as responders, nonresponders, or resistant to aspirin, it is more likely that a spectrum of platelet inhibition is achieved by aspirin.
CLOPIDOGREL RESPONSIVENESS
The standard dose of clopidogrel, much like the standard dose of aspirin, does not exert the same antiplatelet effect in all patients. In a study of 544 patients, Serebrauny et al. evaluated the response to clopidogrel using standard light transmittance aggregometry and flow cytometry (13). Platelet inhibition after clopidogrel therapy, using the change in maximal aggregation by 5 μM ADP, revealed a bell-shaped, normal distribution in which 4.2% of patients were two or more standard deviations above the mean, and 4.8% of patients were at least two standard deviations below the mean, thus creating categories of hyper- and hyporesponders. These findings are consistent with a poly-genetic and poly-environmental influence on the platelet inhibition achieved with clopidogrel therapy, and they suggest that dichotomizing patients into nonresponders and responders is likely a gross oversimplification.
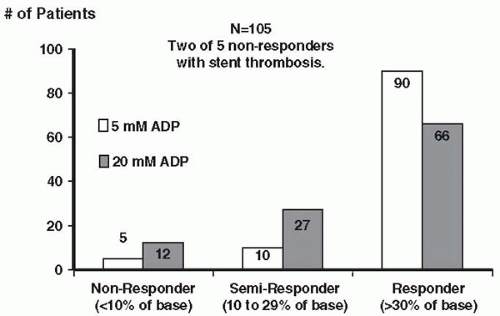
Muller et al. studied 105 aspirin-treated patients with stable coronary artery disease (CAD) who underwent elective PCI. Pretreatment with 600 mg of clopidogrel was given, and platelet inhibition was measured. The rate of clopidogrel nonresponse was 5% with 5 μM ADP and 11% with 20 μM ADP. Of note, among the 105 patients undergoing PCI, stent thrombosis occurred twice, and both patients had been prospectively identified as being clopidogrel nonresponders (Fig. 50.2) (14). These investigators then retrospectively evaluated three other patients who had experienced a stent thrombosis and found all three of these individuals to also be nonresponders.
The baseline level of platelet reactivity is another factor that has been shown to be potentially related to the efficacy of clopidogrel. A study of 92 patients undergoing PCI showed that a higher preprocedural level of platelet
aggregation corresponds to a higher postprocedural level of platelet aggregation (15). At 24 hours postprocedure, P-selectin expression, a marker of platelet activation, was also directly proportional to preprocedural levels. Another study evaluating clopidogrel’s effect on platelet inhibition in PCI patients showed that up to 34% of patients may in fact be nonresponders to clopidogrel at 24 hours after starting the drug. In contrast to the previous study, baseline platelet aggregation was not predictive of clopidogrel response. Patients with significant platelet inhibition at 5 days post-PCI tended to continue their response to clopidogrel at 30 days, thus suggesting a durability in the response to clopidogrel (16).
aggregation corresponds to a higher postprocedural level of platelet aggregation (15). At 24 hours postprocedure, P-selectin expression, a marker of platelet activation, was also directly proportional to preprocedural levels. Another study evaluating clopidogrel’s effect on platelet inhibition in PCI patients showed that up to 34% of patients may in fact be nonresponders to clopidogrel at 24 hours after starting the drug. In contrast to the previous study, baseline platelet aggregation was not predictive of clopidogrel response. Patients with significant platelet inhibition at 5 days post-PCI tended to continue their response to clopidogrel at 30 days, thus suggesting a durability in the response to clopidogrel (16).
GLYCOPROTEIN IIB/IIIA RESPONSIVENESS
Animal studies initially were used to evaluate the doseeffect of GP IIb/IIIa receptor blockade in the setting of arterial injury and demonstrated a wide range in the doses necessary to prevent thrombus formation, which was dependent on the thrombogenic stimulus. An early study utilized a post-thrombolysis reocclusion dog model to evaluate the dose response of monoclonal antiplatelet GP IIb/IIIa antibody 7E3 (23). Only the highest dose (0.8 mg/kg) of 7E3 completely prevented reocclusion. At this dose, >80% of the GP IIb/IIIa receptors were blocked, platelet aggregation induced by 9 μM ADP was completely inhibited, and the bleeding time was prolonged to greater than 30 minutes in most animals. A second study, using the less thrombogenic Foltz model in the monkey carotid artery, showed that only 0.2 mg/kg of 7E3 was required to completely inhibit thrombus formation (24). In contrast, this dose rarely prolonged bleeding time beyond 10 minutes, blocked only ˜70% of GP IIb/IIIa receptors, and inhibited ADP-induced aggregation by ˜70%. The disparity in the levels of platelet inhibition achieved may have been related to an interspecies difference in effects of the monoclonal antibody; however, it is more likely that these results suggest that a higher level of GP IIb/IIIa receptor blockade is required to prevent arterial thrombus formation in the setting of a greater thrombogenic stimulus (25).
Based on the results of these animal models, early dosing of GP IIb/IIIa antagonists was designed to achieve blockade of ˜80% of platelet aggregation to ˜20% of baseline. This dose was considered necessary to prevent ischemic complications in response to severe thrombotic stimuli, but also was recognized as being “highly speculative” (25,26). Several lines of evidence confirm the findings from these early animal studies that a specific level of receptor blockade is necessary to prevent thrombotic complications and suggest that a substantial number of patients may not be achieving this level with standard dosing of GP IIb/IIIa antagonists.
Indirect evidence from several placebo-controlled trials of GP IIb/IIIa antagonists in PCI has suggested the importance of achieving and maintaining a specific level of platelet inhibition in order to minimize thrombotic complications. In the Evaluation of 7E3 for the Prevention of Ischemic Complications (EPIC) trial (27), patients receiving placebo started to require urgent interventions immediately after the initial percutaneous transluminal coronary angioplasty (PTCA), whereas patients receiving only a bolus of abciximab were nearly completely protected for the first 4 to 6 hours (during which time receptor blockade was likely to be ≥80% in most patients), and patients treated with a bolus and 12-hour infusion of abciximab were protected from ischemic complications almost throughout the infusion period. At 30 days, patients who received a bolus and infusion of abciximab had almost a one-third reduction in ischemic events compared with those treated with a bolus alone. This benefit of a bolus and infusion of abciximab versus a bolus alone was maintained for at least 3 years (28,25).
The importance of achieving a high level of receptor blockade with eptifibatide at the time of a PCI is suggested by the discrepant results of two trials utilizing significantly different doses of this agent. In the Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis (IMPACT-II) trial, randomization to a 135 μg/kg bolus and a 0.5 μg/kg per minute infusion of eptifibatide at the time of PCI led to an 18% relative decrease in the composite endpoint compared to placebo (9.2% versus 11.4%, p = 0.063) (29). Later, this dosing regimen was found to produce only ˜50% receptor blockade (30). In the Enhanced Suppression of Platelet Receptor IIb/IIIa Using Integrilin Therapy (ESPIRIT) trial, a much higher dose of eptifibatide was evaluated, with two 180 μg/kg boluses and a 2 μg/kg per minute infusion. In a pilot study, this dose was estimated to provide >90% inhibition of 20 μM ADP-induced aggregation, ˜80% GP IIb/IIIa receptor occupancy, and bleeding times of at least 30 minutes (31). Clinically, this increased dosing regimen was associated with twice the relative benefit of eptifibatide treatment when compared to the results in IMPACT-II, with a 35% reduction in the 30-day composite clinical endpoint of death, MI, or urgent revascularization (6.8% versus 10.4%, p = 0.003) (25).
The Tirofiban and ReoPro Give Similar Efficacy Outcomes Trial (TARGET) compared the efficacy of abciximab versus tirofiban in 4,809 patients undergoing coronary stenting (32). Patients receiving tirofiban had a higher incidence of the primary endpoint of death, nonfatal MI, and urgent target-vessel revascularization (TVR) at 30 days. One potential explanation for these findings is a difference in the level of platelet inhibition achieved with these agents. In a study performed by Hermann et al. (33), platelet inhibition was compared in PCI patients receiving abciximab and tirofiban. The level of platelet inhibition was significantly higher in patients treated with abciximab.
Further clinical trials will have to investigate whether a larger bolus of tirofiban would lead to fewer periprocedural events.
Further clinical trials will have to investigate whether a larger bolus of tirofiban would lead to fewer periprocedural events.
Direct Evidence Correlating Level of Platelet Inhibition with Efficacy Outcomes from Clinical Trials
Several clinical trials have utilized some measure of platelet inhibition and linked these results with efficacy outcomes. In the Platelet IIb/IIIa Antagonism for the Reduction of Acute Coronary Syndrome Events in a Global Organization Network (PARAGON-A) trial, 2,282 patients with a non-ST-elevation acute coronary syndrome were randomized to either a low- or high-dose lamifiban regimen, with or without heparin (38). In an attempt to establish a dose-response relationship, steady-state plasma concentrations of lamifiban were determined in a subgroup of 810 lamifiban-treated patients. Interestingly, a U-shaped correlation was found between plasma drug levels and the incidence of death and MI at 6 months (39). Whereas patients achieving a mid-range of concentrations, representing 80% to 90% GP IIb/IIIa receptor occupancy, realized a 40% relative reduction in death and MI at 30 days, and 38% at 6 months compared with placebo, higher and lower steady-state plasma levels were associated with no significant benefit over placebo (25).
The PARAGON-B trial built upon the knowledge gained from the results of the PARAGON-A study by attempting to carefully target drug levels in a similar patient population (40). Of the 5,225 patients randomized, steady-state plasma concentrations of lamifiban were available in 1,272 patients (41). Interestingly, in contrast to the PARAGON-A results, a U-shaped dose response was not seen, with a trend observed toward greater benefit with increasing plasma levels in the overall PARAGON-B population. The importance of achieving an adequate level of inhibition to optimize clinical outcomes was highlighted by the results in the subgroup of troponin-positive patients, in which patients with low plasma levels derived no benefit compared with patients randomized to placebo, whereas patients with target levels or greater of inhibition experienced a >50% relative decrease in ischemic events (25).
The AU-Assessing Ultegra (GOLD) study prospectively evaluated the level of platelet inhibition achieved with GP IIb/IIIa antagonists and clinical outcomes (42). In this multicenter study, 500 patients undergoing a PCI with the planned use of a GP IIb/IIIa inhibitor had platelet inhibition measured using the point-of-care Ultegra-Rapid Platelet Function Assay (RPFA) (see section, Ultegra-Rapid Platelet Function Assay). The most important finding from this study was that approximately one-quarter of all patients did not achieve ≥95% inhibition 10 minutes following the bolus, and that this lower level of inhibition was associated with a significantly higher incidence of major adverse cardiac events (MACE) (14.4% versus 6.4%, p = 0.006). By multivariate analysis, platelet function inhibition of ≥95% at 10 minutes following the start of therapy was associated with a significant decrease in the incidence of a MACE (odds ratio 0.46, 95%, confidence interval 0.22 to 0.96; p = 0.04) (Fig. 50.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree