History
No
Yes
Comments
Previous AV access
Previous central catheter, PICC line, pacemaker, or defibrillator
Previous venous disease (DVT, superficial thrombophlebitis)
Intravenous drug abuse
Arm disability, relevant activities
Previous surgery
Heart disease or diabetes mellitus
Anticoagulant therapy or coagulation disorder
Dominant hand
Right
Left
Physical
A complete physical examination should be performed giving particular attention to the vascular system. To evaluate the arterial system, bilateral blood pressures and pulses should be assessed along with an Allen’s test to check for an incomplete palmar arch. A blood pressure difference greater than 15 mmHg between arms is significant and should warrant further testing if the arm with the lower pressure is planned for access placement. In most cases, the arm with the higher blood pressure should be used. Tissue loss in the finger tips or hand should also be noted as this finding could indicate underlying arterial occlusive disease. Since hand perfusion will decrease after access placement, any tissue loss should be resolved before surgery. Venous obstruction can manifest as edema, discoloration, collateral veins on the arms or chest, or a difference in arm size. The cardiopulmonary systems should be evaluated for signs of heart failure. Table 18.2 summarizes the pertinent components of the physical examination.
Table 18.2
Physical exam
Physical exam (vascular) | Right | Left | Comments |
Bilateral blood pressures | |||
Brachial | |||
Ankle | |||
Peripheral pulses, +/− Doppler | |||
Carotid | |||
Brachial | |||
Radial | |||
Ulnar | |||
Femoral | |||
Popliteal | |||
Posterior tibial | |||
Dorsal pedal | |||
Allen’s test | nl/abnl | nl/abnl | |
Tissue loss | yes/no | yes/no | |
Edema | yes/no | yes/no | |
Discoloration | yes/no | yes/no | |
Collateral veins | yes/no | yes/no | |
Arm size | nl/abnl | nl/abnl | |
Physical exam (other) | Comments | ||
Cardiac | nl/abnl | ||
Pulmonary | nl/abnl | ||
Imaging
Ultrasound Vein Mapping
As a cost-effective, noninvasive exam that does not require contrast, duplex ultrasound is an ideal imaging modality for evaluating possible autogenous access sites in patients with chronic kidney disease. In addition to measuring the diameter, superficial veins should be assessed for large branches, thrombosis, and thickened walls (evidence of previous phlebitis). The deep venous system should also be evaluated for deep venous thrombosis. A sample vein mapping worksheet is shown (Fig. 18.1) summarizing diameters at multiple points along the upper extremity. In general, a vein diameter of at least 3 mm is preferred for establishing a native arteriovenous fistula.
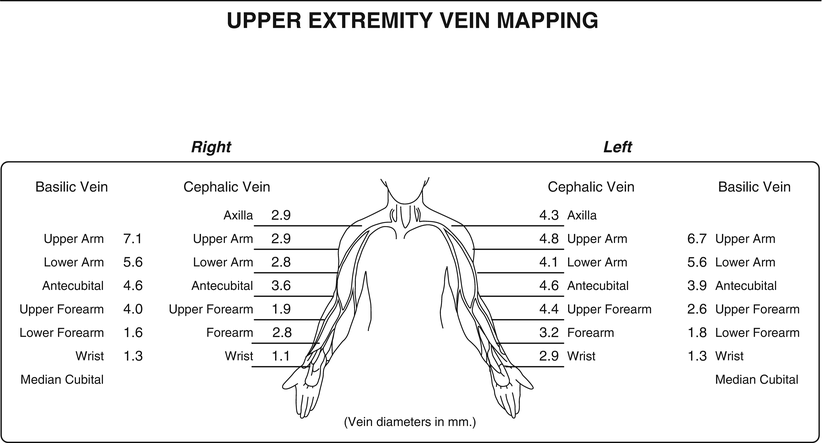

Fig. 18.1
Vein mapping worksheets showing a typical upper extremity mapping of the bilateral basilic and cephalic veins. Note multiple locations for sequential diameters recorded
Venography
Venography should not be routinely performed (especially in patients not on dialysis yet) because it is an invasive exam that requires contrast. Patients who have a pacemaker or a history of central venous catheters (especially subclavian), as well as those with signs of central venous stenosis, should be considered for venography to assess the central veins. Venography to evaluate the veins in the upper extremity should be used selectively.
Order of Site Preference Principles and Guidelines
Fistula First, Catheter Last Principles
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) ranks the order in which AV access for dialysis should be attempted, which follows the principles of “fistula first, catheter last”. The recommendations generally describe placement of autogenous AV fistulas, followed by grafts in distal to proximal locations in the upper extremities. The first choice is a “snuffbox” radial-cephalic AV fistula followed by more proximal upper extremity AV fistulas, then AV grafts. Once all upper extremity locations have been used, alternative sites, such as axillary and lower extremity fistulas may be considered. Catheters are used for permanent hemodialysis access as a last resort. These guidelines are based on the survival and safety advantages of autogenous AV fistulas over AV grafts and catheters. The order of preferences is summarized in Table 18.3 [1].
Table 18.3
Order of preference guidelines for AV access
Fistula/Graft type | Advantages | Drawbacks |
|---|---|---|
Preferred | ||
Radial-cephalic fistula (snuffbox followed by wrist) | The preferred first location, straightforward procedure, preserves proximal sites | Lower flow, slower maturation, higher risk of hand ischemia |
Brachial-cephalic fistula | Higher flow, more reliable maturation | Increased incidence of edema and ischemic steal syndrome may require superficialization |
Brachial-basilic (transposition) fistula | Less likely to have been previously accessed due to its deeper and more medial location | Increased pain and edema postop, increased risk of ischemic steal syndrome, kinking if tunneling, requires transposition of length of basilic vein, technically challenging in obese |
Acceptable | ||
Forearm loop AV graft | Shorter lag time, easy cannulation | Infection, thrombosis, postop pain and edema, lower flow than more proximal grafts |
Upper arm AV graft (straight or curved followed by loop) | Higher blood flow than distal grafts | Infection, increased risk of ischemic steal syndrome |
Femoral AV graft | High blood flow | Infection, ischemia, difficulty positioning for dialysis, difficult if obese |
Necklace AV graft | Infection, thrombosis, not for patients who need sternotomy | |
Avoid if possible | ||
Tunneled HD catheter | Immediate access | Last resort due to infection and thrombosis |
Advantages and Drawbacks of Autogenous AV Fistulas
Patients with autogenous AV fistulas have safer, more effective dialysis and live longer than patients with AV grafts and catheters. AV fistulas are superior to grafts and catheters with regard to infection rate, thrombosis rate, long-term patency, and cost [2]. A drawback of autogenous AV fistulas is their maturation time of at least 6 weeks compared to grafts which require 2 weeks and catheters which are ready for use immediately. Autogenous AV fistulas also have a higher primary failure rate, and some fistulas never mature. Balloon angioplasty maturation (BAM) has emerged as a promising technique to improve maturation of small caliber autogenous AV fistulas. BAM involves sequential dilation to create a controlled rupture of the vein which then remodels into a large caliber vascular conduit [3].
Technical Details
General Operative Considerations
Anesthesia
AV access placement can be done under general anesthesia, regional block, or local anesthesia with sedation. The author (VG) uses the latter for the vast majority of primary procedures. General anesthesia or regional blocks are generally used for more complex or redo procedures. Selected patients with complicating conditions (e.g., claustrophobia, chronic back pain, psychologic disturbances) may require general anesthesia.
Heparin
Evidence supporting a clear benefit for intraoperative heparin during AV fistula surgery remains elusive. Some studies showed no difference in bleeding complications, and 30-day patency rates with systemic heparin administration [4] and others demonstrated an increased risk of bleeding with heparinization and no benefit in terms of primary patency [5]. The use of systemic heparin in the creation of AV fistulas is therefore based on surgeon preference and experience.
Tunneling
To avoid kinking or twisting of the vein, proper orientation should be maintained by marking the vessel along one surface prior to tunneling. The vein should be tunneled superficially to make it easier to define and puncture for dialysis. It is important to note that the position of the patient’s arm during the operation (abduction) is usually different than the more anterior position of the arm during dialysis. Unimpeded access should be possible when the arm is in a natural position for the patient. Access placement issues become more important in patients with redundant tissue or decreased mobility.
Anastomosis
The anastomotic diameter should be limited to minimize the risk of hemodynamic steal syndrome. Although they are not definitive, guidelines for the size of the anastomosis have been proposed. For the brachial artery, the anastomotic diameter should be 4–6 mm [6, 7]. For radial artery fistulas, the anastomotic diameter should be between 5 and 8 mm [8].
Thrill/Pulse
The presence of a thrill over the new AV fistula should be noted both before and after closure of the incisions. A distal pulse should be palpated. If it is not palpable, the pulse should be reevaluated during manual compression of the AVF. If the pulse returns with AVF compression, then the arterial flow is intact. If the pulse does not return, further investigation is warranted for arterial thrombosis or embolus. At a minimum, good distal Doppler signals and capillary refill should be present before leaving the operating room.
Surgical Exposures
This section describes the common exposures for isolating the radial, brachial, axillary, and femoral vessels.
Radial Artery
A longitudinal incision is made along the lateral wrist over or just lateral to the radial pulse (Fig. 18.2). The radial artery is located just lateral to the flexor carpi radialis tendon.
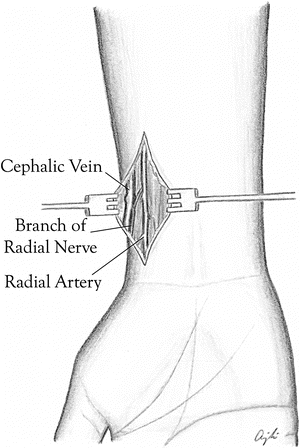

Fig. 18.2
Wrist dissection showing the main structures of the radial artery, cephalic vein, and branch of the radial nerve
Brachial Artery and Vein
Brachial artery exposure typically involves a transverse incision just distal to the antecubital crease (Fig. 18.3a, b). To expose the brachial artery, the aponeurosis of the biceps tendon is partially divided. The brachial artery should be isolated both proximally and distally with vessel loops. Small arterial branches should also be identified and isolated with vessel loops. The nerve closest to the brachial artery is the median nerve, which is medial to the vessels. This nerve is more prominent during exposure proximal to the antecubital crease.
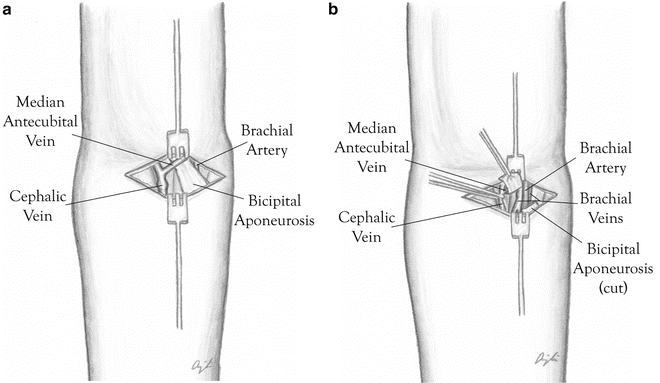

Fig. 18.3
(a) Transverse incision just distal to the antecubital crease showing the cephalic vein, median cubital vein, and the bicipital aponeurosis overlying the brachial artery and vein. (b) Dissection carried deeper with transaction of the bicipital aponeurosis and exposure of the brachial artery and veins
For dissection proximal to the antecubital crease, the patient’s arm is abducted to 90°. A longitudinal incision is made on the medial arm over the groove between the biceps and triceps muscles (Fig. 18.4). The basilic vein can be visualized medial to the brachial sheath. The median and ulnar nerves are usually encountered during the dissection.
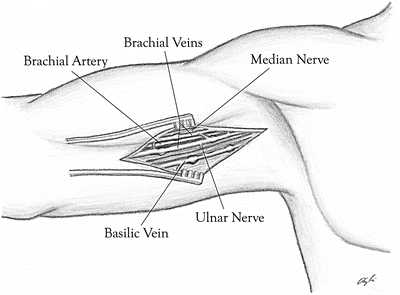

Fig. 18.4
The brachial artery is dissected out proximally. Note the brachial artery and surrounding brachial veins with crossing branches. The median nerve and ulnar nerves can be exposed in this dissection
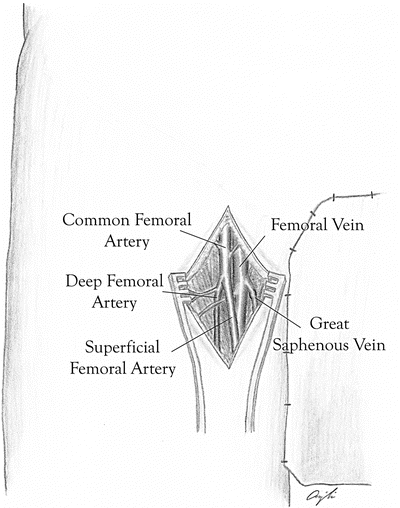
Femoral Artery and Vein
A longitudinal or oblique incision should be made just distal to the inguinal ligament (Fig. 18.5). The dissection is carried down to the common femoral artery. The femoral bifurcation is identified and isolated. The dissection is then continued medially to expose the femoral veins. The common femoral, deep femoral, proximal femoral, and saphenofemoral junction are isolated and controlled. The femoral nerve is lateral to the artery and should not be visualized during the standard dissection.