After reading this chapter, you will be able to: • Identify the following respiratory acid-base disturbances: • Acute alveolar hyperventilation (acute respiratory alkalosis) • Acute alveolar hyperventilation with partial renal compensation (partially compensated respiratory alkalosis) • Chronic alveolar hyperventilation with complete renal compensation (compensated respiratory alkalosis) • Acute ventilatory failure (acute respiratory acidosis) • Acute ventilatory failure with partial renal compensation (partially compensated respiratory acidosis) • Chronic ventilatory failure with complete renal compensation (compensated respiratory acidosis) • Acute alveolar hyperventilation superimposed on chronic ventilatory failure • Acute ventilatory failure superimposed on chronic ventilatory failure • Identify the following metabolic acid-base disturbances: • Metabolic acidosis with partial respiratory compensation • Metabolic acidosis with complete respiratory compensation • Metabolic alkalosis with partial respiratory compensation • Metabolic alkalosis with complete respiratory compensation • Identify the following combined acid-base disturbances: • Combined metabolic and respiratory acidosis • Combined metabolic and respiratory alkalosis • Describe the pH, Paco2, and • How acute Pco2 increases affect the pH and • How acute Pco2 decreases affect the pH and • The quick clinical calculation commonly used for acute Pco2 changes on pH and • The general rule of thumb for the Paco2/ • Describe the following six most common acid-base abnormalities seen in the clinical setting: • Acute alveolar hyperventilation (acute respiratory alkalosis) • Acute ventilatory failure (acute respiratory acidosis) • Chronic ventilatory failure (compensated respiratory acidosis) • Acute alveolar hyperventilation superimposed on chronic ventilatory failure • Acute ventilatory failure superimposed on chronic ventilatory failure • Lactic acidosis (metabolic acidosis) • Describe the metabolic acid-base abnormalities including metabolic acidosis, anion gap, and metabolic alkalosis. • List the causes of metabolic acidosis and metabolic alkalosis. • Describe the potential hazards of oxygen therapy in patients with chronic ventilatory failure with hypoxemia. • Define key terms and complete self-assessment questions at the end of the chapter and on Evolve. As the pathologic processes of a respiratory disorder intensify, the patient’s arterial blood gas (ABG) values are usually altered to some degree. Table 4-1 lists the normal ABG values. Box 4-1 provides an overview of the respiratory and metabolic acid-base disturbances. In the profession of respiratory care, a basic knowledge and understanding of the acid-base disturbances is an absolute—and unconditional—prerequisite to the assessment and treatment of the patient with a respiratory disorder. Because of the fundamental importance of this subject, this chapter provides the following review: Table 4-1 *Technically, only the oxygen (Po2) and carbon dioxide (Pco2) pressure readings are true blood gas values. The pH indicates the balance between the bases and acids in the blood. The bicarbonate ( • The Pco2/ • The six most common acid-base abnormalities seen in the clinical setting • The metabolic acid-base abnormalities • The hazards of oxygen therapy in patients with chronic ventilatory failure with hypoxemia To fully understand the clinical significance of the acid-base disturbances listed in Box 4-1, a fundamental knowledge base of the Pco2/ The thick red bar moving from left to right across the Pco2/ Although it is beyond the scope of this textbook to fully explain how each of the acid-base disturbances listed in Box 4-1 can be identified on the Pco2/ As mentioned previously, the red normal Pco2 blood buffer bar shown on the Pco2/ On the other hand, the red normal Pco2 blood buffer bar shown on the Pco2/ In addition to using the graphic Pco2/
Arterial Blood Gas Assessments
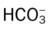 relationship, and include the following:
relationship, and include the following:
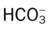 values
values
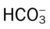 values
values
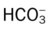 values
values
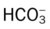 /pH relationship
/pH relationship
Acid-Base Abnormalities
Blood Gas Value*
Arterial
Venous
pH
7.35 to 7.45
7.30 to 7.40
Pco2
35 to 45 mm Hg
42 to 48 mm Hg
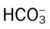
22 to 28 mEq/L
24 to 30 mEq/L
Po2
80 to 100 mm Hg
35 to 45 mm Hg

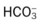 ) reading is an indirect measurement that is calculated from the pH and Pco2 levels.
) reading is an indirect measurement that is calculated from the pH and Pco2 levels.
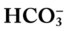 /pH relationship—an essential cornerstone of ABG interpretations
/pH relationship—an essential cornerstone of ABG interpretations
The PCO2/
 /pH Relationship
/pH Relationship
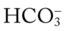 /pH relationship is essential. The Pco2/
/pH relationship is essential. The Pco2/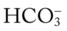 /pH relationship is graphically illustrated in the
/pH relationship is graphically illustrated in the  nomogram shown in Figure 4-1.*
nomogram shown in Figure 4-1.*
How to Read the PCO2/
 /pH Nomogram
/pH Nomogram
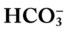 /pH nomogram represents the normal Pco2 blood buffer line. This red bar is used to identify the pH and
/pH nomogram represents the normal Pco2 blood buffer line. This red bar is used to identify the pH and 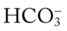 changes that occur immediately in response to an acute increase or decrease in Pco2. The purple bar is used to identify the pH and
changes that occur immediately in response to an acute increase or decrease in Pco2. The purple bar is used to identify the pH and 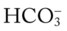 changes that occur in response to acute metabolic acidosis and metabolic alkalosis conditions. The colored areas that surround the red and purple bars are used to identify (1) partial and complete renal compensation, (2) partial and complete respiratory compensation, and (3) combined metabolic and respiratory acid-base disturbances (see Figure 4-1).
changes that occur in response to acute metabolic acidosis and metabolic alkalosis conditions. The colored areas that surround the red and purple bars are used to identify (1) partial and complete renal compensation, (2) partial and complete respiratory compensation, and (3) combined metabolic and respiratory acid-base disturbances (see Figure 4-1).
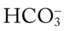 /pH nomogram, a basic understanding of the following two most commonly encountered Pco2/
/pH nomogram, a basic understanding of the following two most commonly encountered Pco2/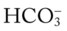 /pH relationships is important: (1) an acute Pco2 increase and its effects on the pH and
/pH relationships is important: (1) an acute Pco2 increase and its effects on the pH and 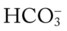 values, and (2) an acute Pco2 decrease and its effects on the pH and
values, and (2) an acute Pco2 decrease and its effects on the pH and 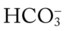 values.*
values.*
How Acute PCO2 Increases Affect the pH and
 Values
Values
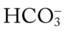 /pH nomogram is used to identify the pH and
/pH nomogram is used to identify the pH and 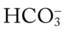 values that will result immediately in response to a sudden increase in Pco2. For example, if the patient’s Paco2 were to suddenly increase to, say, 60 mm Hg, the pH would immediately fall to about 7.28 and the
values that will result immediately in response to a sudden increase in Pco2. For example, if the patient’s Paco2 were to suddenly increase to, say, 60 mm Hg, the pH would immediately fall to about 7.28 and the 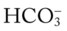 level would increase to about 26 mEq/L. Furthermore, the Pco2/
level would increase to about 26 mEq/L. Furthermore, the Pco2/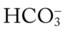 /pH nomogram shows that these ABG values represent acute ventilatory failure (acute respiratory acidosis). This is because (1) all of the ABG values (i.e., Pco2,
/pH nomogram shows that these ABG values represent acute ventilatory failure (acute respiratory acidosis). This is because (1) all of the ABG values (i.e., Pco2, 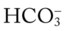 , and pH) intersect within the red normal Pco2 blood buffer bar, and (2) the pH and
, and pH) intersect within the red normal Pco2 blood buffer bar, and (2) the pH and 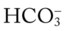 readings are precisely what is expected for an acute increase in the Pco2 of 60 mm Hg (Figure 4-2).
readings are precisely what is expected for an acute increase in the Pco2 of 60 mm Hg (Figure 4-2).
How Acute PCO2 Decreases Affect the pH and
 Values
Values
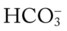 /pH nomogram is also used to identify the pH and
/pH nomogram is also used to identify the pH and 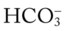 values that will result immediately in response to a sudden decrease in Pco2. For example, if the patient’s Paco2 were suddenly to decrease to, say, 25 mm Hg, the pH would immediately increase to about 7.55 and the
values that will result immediately in response to a sudden decrease in Pco2. For example, if the patient’s Paco2 were suddenly to decrease to, say, 25 mm Hg, the pH would immediately increase to about 7.55 and the 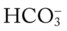 level would decrease to about 21 mEq/L. In addition, the Pco2/
level would decrease to about 21 mEq/L. In addition, the Pco2/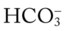 /pH nomogram shows that these ABG values represent acute alveolar hyperventilation (acute respiratory alkalosis). This is because (1) all of the ABG values (i.e., Pco2,
/pH nomogram shows that these ABG values represent acute alveolar hyperventilation (acute respiratory alkalosis). This is because (1) all of the ABG values (i.e., Pco2, 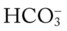 , and pH) intersect within the red normal Pco2 blood buffer bar, and (2) the pH and
, and pH) intersect within the red normal Pco2 blood buffer bar, and (2) the pH and 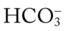 readings are precisely what is expected for an acute increase in the Pco2 of 25 mm Hg (Figure 4-3).
readings are precisely what is expected for an acute increase in the Pco2 of 25 mm Hg (Figure 4-3).
A Quick Clinical Calculation for the Effect of Acute PaCO2 Changes on pH and

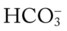 /pH nomogram (see Figure 4-1), the following simple calculations can also be used to estimate the expected pH and
/pH nomogram (see Figure 4-1), the following simple calculations can also be used to estimate the expected pH and 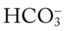 value changes that will immediately occur in response to a sudden increase or decrease in Paco2.
value changes that will immediately occur in response to a sudden increase or decrease in Paco2.

 nomogram
nomogram relationship
relationship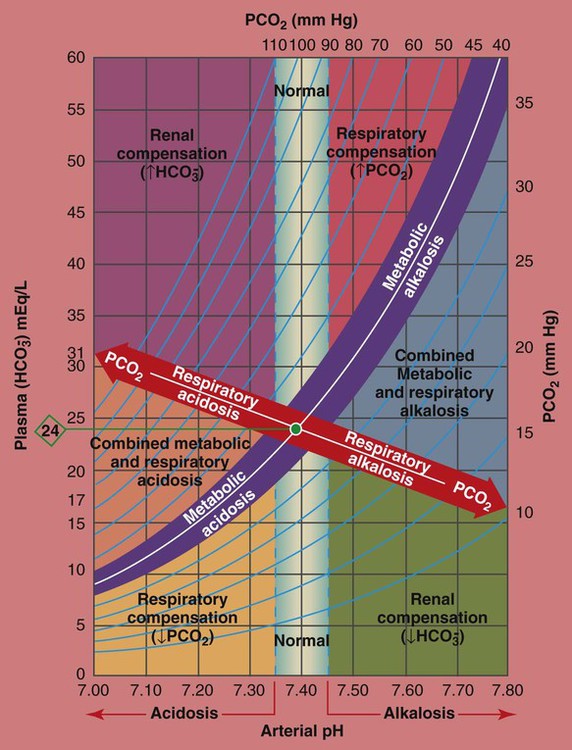
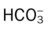 /pH relationship. For explanation see text. (Used with permission from author, Terry DesJardins.)
/pH relationship. For explanation see text. (Used with permission from author, Terry DesJardins.)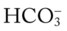 values all intersect in the light purple area—shown in the upper left hand corner of the P
values all intersect in the light purple area—shown in the upper left hand corner of the P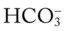 /pH nomogram—partial renal compensation has occurred in response to a chronically high P
/pH nomogram—partial renal compensation has occurred in response to a chronically high P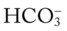 increases enough to move the pH into the light-blue normal bar, complete renal compensation is confirmed. When the pH, P
increases enough to move the pH into the light-blue normal bar, complete renal compensation is confirmed. When the pH, P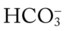 values all intersect in the in the green area—shown in the lower right hand corner of the P
values all intersect in the in the green area—shown in the lower right hand corner of the P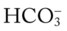 /pH nomogram—partial renal compensation has occurred in response to a chronically low P
/pH nomogram—partial renal compensation has occurred in response to a chronically low P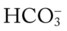 decreases enough to move the pH into the light-blue normal bar, complete renal compensation is confirmed.
decreases enough to move the pH into the light-blue normal bar, complete renal compensation is confirmed.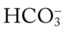 values all intersect in the in the orange area—shown immediately below the red bar on the left side of the P
values all intersect in the in the orange area—shown immediately below the red bar on the left side of the P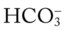 /pH nomogram—a combined respiratory and metabolic acidosis is confirmed. When the pH, P
/pH nomogram—a combined respiratory and metabolic acidosis is confirmed. When the pH, P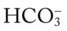 values all intersect in the in the blue area—shown immediately above the red bar on the right side of the P
values all intersect in the in the blue area—shown immediately above the red bar on the right side of the P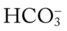 /pH nomogram—a combined respiratory and metabolic alkalosis is confirmed.
/pH nomogram—a combined respiratory and metabolic alkalosis is confirmed.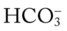 values all intersect in the yellow area—shown in the lower left corner of the P
values all intersect in the yellow area—shown in the lower left corner of the P /pH nomogram—respiratory compensation has occurred in response to metabolic acidosis. When the pH, P
/pH nomogram—respiratory compensation has occurred in response to metabolic acidosis. When the pH, P values all intersect in the pink area—shown in the upper right corner of the P
values all intersect in the pink area—shown in the upper right corner of the P /pH nomogram—respiratory compensation has occurred in response to metabolic alkalosis.
/pH nomogram—respiratory compensation has occurred in response to metabolic alkalosis.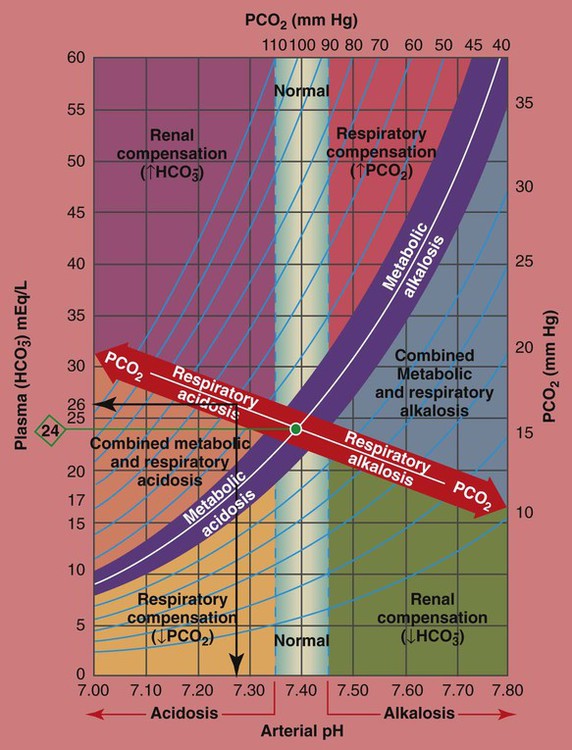
 values all intersect within the red-colored respiratory acidosis bar to the left of the light blue, vertical bar labeled “normal”. For example, when the P
values all intersect within the red-colored respiratory acidosis bar to the left of the light blue, vertical bar labeled “normal”. For example, when the P is 26 mEq/L, acute ventilatory failure is confirmed. (Used with permission from author, Terry DesJardins.)
is 26 mEq/L, acute ventilatory failure is confirmed. (Used with permission from author, Terry DesJardins.)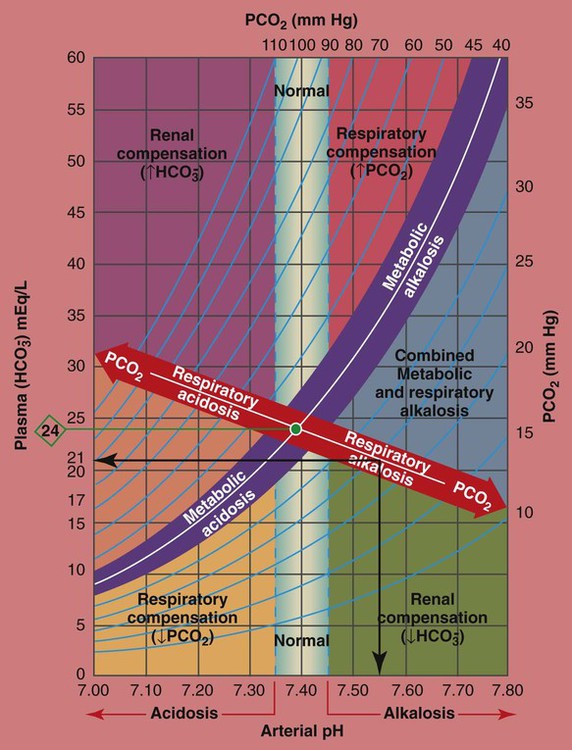
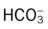 values all intersect within the red-colored “Respiratory alkalosis” bar. For example, when the reported P
values all intersect within the red-colored “Respiratory alkalosis” bar. For example, when the reported P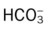 is 21 mEq/L, acute alveolar hyperventilation is confirmed. (Used with permission from author, Terry DesJardins.)
is 21 mEq/L, acute alveolar hyperventilation is confirmed. (Used with permission from author, Terry DesJardins.)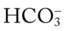 24 mEq/L), for every 10 mm Hg the Pa
24 mEq/L), for every 10 mm Hg the Pa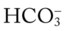 will increase about 1 mEq/L (from 24). Or, by way of another example, for every 20 mm Hg the Pa
will increase about 1 mEq/L (from 24). Or, by way of another example, for every 20 mm Hg the Pa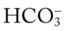 will increase about
will increase about 

