Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia
Peter T. Buser
Michael Zellweger
Jens Bremerich
CLINICAL BACKGROUND
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a predominantly genetically determined and inheritable disease characterized by fibrofatty replacement of the right ventricular myocardium with ventricular arrhythmias and sudden death (1,2). Its prevalence has been estimated to range from 1 in 2,000 to 1 in 5,000, and men are more frequently affected than women with a ratio of 3:1. It has been reported to account for 3% to 10% of unexplained sudden cardiac death in patients less than 65 years old (3,4). Patients with symptomatic ARVC may therefore be candidates for an active therapeutic management, including antiarrhythmic medication, invasive electrophysiologic procedures, implantation of a cardioverter-defibrillator, and in some cases, cardiac transplantation (5).
It is a rare disorder, but it is the most common cause of sudden cardiac death in younger populations. Major pathologic findings are replacement of the myocardium by fatty and fibrous tissue and numerous structural abnormalities of the right ventricle including areas of wall thinning with formation of single or multiple aneurysms. These abnormalities are most often found in the subtricuspid area, the right ventricular apex, and the right ventricular outflow tract— the so-called triangle of ARVC. However, left ventricular involvement has been described especially in the posterolateral wall (6), may be age dependent and associated with arrhythmic events, cardiomegaly, and heart failure. Twelve genes have been identified which are linked to ARVC, encoding several components of the cardiac desmosome. Dysfunction of desmosomes causes defective cell adhesion, loss of electrical myocyte coupling leading to apoptotic myocyte death, fibrofatty replacement, and arrhythmias. Clinically, four stages of the disease have been proposed: (a) A concealed phase, in which anatomic changes are subtle and arrhythmias may be minor, but in which sudden cardiac death may be the first indication of the disease; (b) an overt electrical disorder presenting with ventricular tachycardias or sudden cardiac death, in which structural and functional abnormalities of the right ventricle are more apparent; (c) progressive right ventricular failure with preserved left ventricular function; and (d) biventricular dysfunction (7).
The diagnosis of ARVC can be made according to the International Task Force criteria, originally published in 1994 (8) and modified in 2010 (9). Abnormalities are divided into major and minor categories according to their specificity for ARVC. Criteria for global and regional dysfunction and/or structural alterations of the right ventricle are based on findings with transthoracic echocardiography or cardiac magnetic resonance (CMR) imaging. Further criteria include tissue characterization of myocardial walls by endomyocardial biopsy and histology, repolarization and depolarization abnormalities by ECG, arrhythmias by (Holter-) monitoring, and family history with pathologically proven ARVC. The modified ARVC Task Force criteria provide detailed cutoffs for abnormal RV size and wall motion as assessed by CMR. RV ejection fraction ≤40%, or regional akinesia, dyssynchronous RV contraction, RV end-diastolic volume/body surface area ≥110 mL/m2 (male) or ≥100 mL/m2 (female) are considered major criteria for ARVC.
CMR has the unique advantage to be an absolutely noninvasive technique, providing information on dimensions, geometry, shape, volumes, and function of all cardiac chambers, tissue characterization such as differentiation of fat from myocardium, and identification of fibrous formations such as myocardial scar. CMR, therefore, has been increasingly used for the evaluation of right ventricular disease and has evolved as the noninvasive imaging modality of choice in ARVC.
CARDIAC MAGNETIC RESONANCE TECHNIQUES
There is no widely accepted standard imaging protocol for the investigation of patients with suspected ARVC using CMR. The CMR imaging protocol is aimed at recognizing both, typical features of ARVC such as RV akinesia, dyskinesia, dyssynchrony, and reduced RV ejection fraction (modification of ARVC/D Task Force criteria) as well as features of other conditions with similar clinical presentation, for example, granulomas in cardiac sarcoidosis. We suggest an imaging protocol that comprises cine steady-state free precession (Cine-bSSFP) for morphology and function, T1-weighted turbo-spin-echo (T1-TSE) for morphology, and late gadolinium enhancement inversion recovery gradient recalled echo (LGE-IR-GRE) sequences acquired in axial and short-axis orientation. The right ventricle has a complex geometry, is asymmetric, and is highly trabeculated. The mean right ventricular free wall thickness in healthy individuals is 2.7 ± 0.4 mm and only 1.9 ± 1.1 mm at the right ventricular apex (8). Epicardial fat is usually present, especially in association with the right coronary artery within the right atrioventricular groove and with the left anterior descending coronary artery in the apical region. Tongues of epicardial fat may extend into the myocardium in healthy individuals (10,11). The identification of small morphologic alterations of the thin right ventricular free wall, the analysis of regional wall-motion abnormalities of a complex geometric structure, and the identification of intramural or transmural fatty infiltrates of the right ventricular myocardium require highresolution images without significant artifacts. Retrospective analysis of static magnetic resonance images of 39 patients from a ARVC registry revealed an excellent image quality in less than 10% of cases (12). Thus, basic requirements for equipment, software, imaging protocol, readout, and documentation should be fulfilled for the CMR investigation of patients with suspected ARVC. A cardiac phased array coil is preferred, and gradient coil strength should minimally be 20 mT/m. Cardiac gating and breath-hold imaging are required (13). Black blood imaging is used to depict morphologic abnormalities of the right ventricle and intramyocardial fatty infiltration. Bright blood cine imaging is used for visualizing global and regional ventricular function and to measure end-diastolic and end-systolic volumes for calculation of stroke volume and ejection fraction. In addition, significant valvular disease can be demonstrated or excluded. Diastolic dysfunction of the right ventricle can be assessed by measurement of blood-flow velocities across the tricuspid valve during the whole cardiac cycle by application of MR velocity mapping (14).
BLACK BLOOD IMAGING
For black blood techniques, breath-hold imaging with double-inversion recovery fast spin-echo (DIR-FSE) techniques is preferred to traditional spin-echo (SE) imaging. These techniques substantially shorten imaging time and virtually eliminate respiratory motion artifacts. Black blood inversion prepared, half Fourier single-shot turbo spin-echo (HASTE) imaging currently is not recommended because of blurring of subtle anatomic details. Frequent ventricular ectopy can substantially deteriorate image quality. In patients with suspected ARVC and frequent ventricular ectopy, a low dose of oral beta-blocker (e.g., 50 mg metoprolol) can be given 1 hour before the CMR examination to reduce ventricular ectopy. However, contraindications for beta-blockade must be considered.
Right ventricular morphology is best shown in axial and sagittal imaging planes. Axial imaging planes should encompass the whole heart starting from the pulmonary artery to the diaphragm. Axial imaging planes provide the best view of the right ventricular anterior wall up to the proximal parts of the right ventricular outflow tract. Since the anatomic course of the right ventricular outflow tract toward the pulmonary valve and the common pulmonary artery progresses from ventral to dorsal, axial planes will not be optimal for the assessment of dimensions and morphology of the subpulmonary part of the right ventricular outflow tract. Sagittal planes through the right ventricular outflow tract will depict this portion optimally.
BRIGHT BLOOD IMAGING
For bright blood imaging, balanced steady-state free precession imaging (FIESTA, true FISP) is the preferred technique because it allows better endocardial definition when compared with spoiled gradient-echo images (FLASH or FASTCARD). Cine imaging in the axial plane is optimal to assess right ventricular global and regional function. Cine imaging in the axial plane is optimal to assess right ventricular global and regional function. Again, the whole heart is covered from the pulmonary artery to the diaphragm. Contraction abnormalities of the right ventricular outflow tract, areas of wall thinning and reduced contraction, aneurysm formation, regions of focal hypokinesia, and akinesia or dyskinesia of the right ventricular free wall can be best depicted in axial imaging planes. However, at least one additional imaging plane should be used to assess function of the diaphragmatic right ventricular wall segments, function of the right ventricular outflow tract, and left ventricular volumes and function. In sagittal imaging planes, the right ventricular outflow tract is usually well depicted along its long axis as well as diaphragmatic segments of the right ventricular wall. In addition, to assess left ventricular regional and global function accurately and to measure right ventricular and left ventricular volumes, proper left ventricular short-axis planes encompassing the whole left ventricle from above the atrioventricular plane to the apex should be used. Quantification of ventricular volumes is performed by contouring the enddiastolic and end-systolic frames of the entire right ventricle and left ventricle, using a summation of disks method (Simpson’s rule) with integration over the image slices. For
further evaluation of left ventricular abnormalities, especially in the anterior and apical segments, two-chamber and/or four-chamber long-axis imaging planes should be added.
further evaluation of left ventricular abnormalities, especially in the anterior and apical segments, two-chamber and/or four-chamber long-axis imaging planes should be added.
INTERPRETATION OF CARDIAC MAGNETIC RESONANCE FINDINGS
RIGHT VENTRICULAR VOLUMES, GLOBAL AND REGIONAL DYSFUNCTION
Regional akinesia or dyskinesia of the right ventricular wall, or dyssynchronous contraction of the right ventricle coinciding with an enlarged right ventricular end-diastolic volume (Figs. 11.1 and 11.2) indexed by body surface area of ≥110 mL/m2 for male and ≥100 mL/m2 for female, or an impaired right ventricular ejection fraction ≤40%, are considered as major criteria for the diagnosis of ARVC according to the revised Task Force criteria (9). Minor criteria are regional akinesia or dyskinesia of the right ventricular wall or dyssynchronous contraction of the right ventricle coinciding with an enlarged right ventricular end-diastolic volume indexed by body surface area of 100 to 110 mL/m2 for male and 90 to 100 mL/m2 for female or an impaired right ventricular ejection fraction between 40% and 45%.
CMR is the gold standard imaging modality for the assessment of cardiac ventricular volumes. Minor changes in ventricular volumes overtime can be detected and provide insight into an evolving disease process. Small increases in right ventricular end-diastolic volumes may be an early sign of ARVC. Auffermann et al. (15) reported an increased right ventricular end-diastolic volume index in 10 patients with ARVC and inducible ventricular arrhythmias. Ventricular volumes of those patients who were not inducible did not differ from those of control subjects. Tandri et al. (13) found that 75% of patients with Task Force criteria for ARVC have some degree of right ventricular enlargement and dysfunction. These abnormalities were confirmed during re-evaluation of 14 patients in whom right ventricular functional abnormalities were noted on CMR performed at initial evaluation. Furthermore, all of these patients met the Task Force criteria for ARVC during re-evaluation (16).
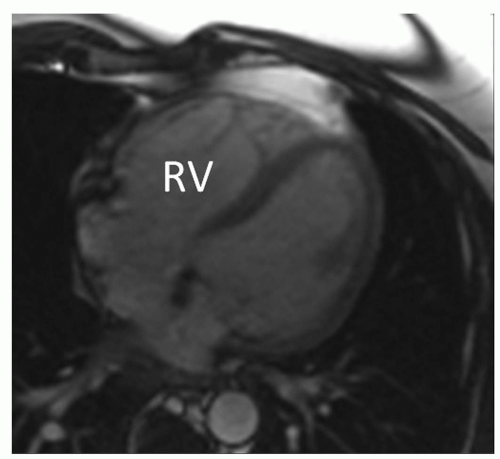 Figure 11.1. Bright blood image from a cine loop in an axial plane at end-diastole in a patient with ARVC. Enlargement of the RV, thinning of the RV free myocardial wall. |
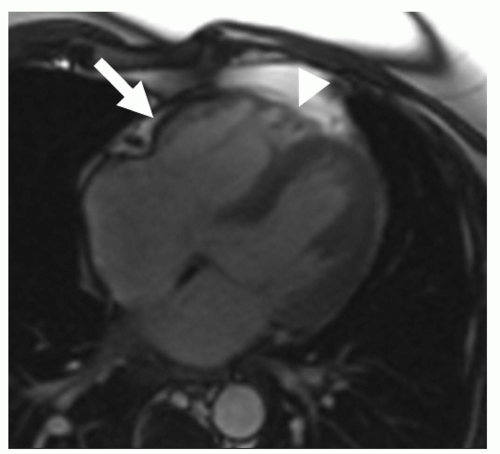 Figure 11.2. Bright blood image from a cine loop in an axial plane at end-systole in a patient with ARVC. Aneurysm in the subtricuspid area (arrow), microaneurysms in the RV apex (triangular mark). |
High-resolution CMR cine imaging with state of the art SSFP sequences is considered as gold standard for the assessment of ventricular volumes, myocardial mass, and systolic function with high intra- and interobserver agreement and accuracy (17,18). Regional abnormalities of right ventricular function are thought to precede global dysfunction. These include regional hypokinesia, defined as systolic wall thickening less than 40%, and akinesia, defined as systolic wall thickening less than 10% because of myocyte loss, fibrosis, and impaired myocardial contraction. Dyskinesia, defined as systolic outward movement, and bulging during diastole are signs of aneurysm formation. Several studies have consistently reported a high incidence of right ventricular regional dysfunction in ARVC (13,15,19,20,21,22 and 23). Tandri et al. (13




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


