(1)
University of Ottawa The Ottawa Hospital, Ottawa, ON, Canada
The rational basis of antiarrhythmic therapy ideally requires a knowledge of:
1.
The mechanism of the arrhythmia:
Disturbances in impulse generation (enhanced automaticity or ectopic tachyarrhythmia).
Disturbances of impulse conduction (reentrant arrhythmias). Most of the evidence suggests that reentry is the mechanism for sustained ventricular tachycardia (VT).
The site or sites where such disturbances are present.
2.
The mode of action of the drug with which control of the arrhythmia is to be attempted
3.
The clinical situation:
Acute, persistent, or paroxysmal
Associated with a low blood pressure <90 mmHg, congestive heart failure (HF), or distressing symptoms
Associated with cardiac pathology, accessory pathway or secondary to hypoxemia, acute blood loss, electrolyte or acid-base imbalance, extracardiac conditions as diverse as thyrotoxicosis, chronic obstructive pulmonary disease, or acute conditions such as pyrexial illness, pneumothorax, or even rupture of the esophagus
Prevention of episodes of arrhythmia and treatment of the acute attack are considered separately.
Except in emergency, the first step in therapy is to remove or correct a precipitating cause when this is feasible, thus reducing the need for antiarrhythmic medication. Precipitating factors include heart failure (HF), ischemia, digoxin toxicity or administration of beta-stimulants or theophylline, sick sinus syndrome, atrioventricular (AV) block, thyrotoxicosis, hypokalemia, hypomagnesemia, hypoxemia, acute blood loss, acid-base disturbances, and infection.
Not all arrhythmias require drug treatment, and the need for therapy is considered carefully for each individual bearing in mind the limited effectiveness of drugs and the occurrence of adverse effects, which is particularly important during long-term management and the occasional unexpected proarrhythmic effect of some drugs.
In addition, especially in the management of ventricular arrhythmias that are difficult to control, it is important to achieve target plasma concentrations known to be associated with suppression of the arrhythmia. This requires careful titration of dosage dictated by salutary effects and sometimes by concentration monitoring.
Note: Plasma concentration for some drugs does not reflect the full activity of the drug or metabolites.
Classification
Antiarrhythmic drugs may be considered from three distinct points of view:
1.
According to their site of action.
2.
According to their electrophysiologic action on isolated cardiac fibers [classes I–IV, as proposed by Vaughan Williams (1970)].
Class IA (quinidine, disopyramide, procainamide) are membrane stabilizing; they inhibit fast sodium channel causing restriction of sodium current and slowing phase 0 of the action potential; the duration of the action potential is thus slightly prolonged.
IB drugs (lidocaine, mexiletine) are rapidly attached to sodium channels during the action potential. Thus, few channels are available for activation at the commencement of diastole, and the effective refractory period (ERP) is prolonged. During diastole, however, the drugs are rapidly detached. At the end of diastole, most channels are drug free. Thus, there is no slowing of conduction velocity in the ventricle or His–Purkinje system. There is minimal slowing of phase 0.
Class IC drugs, flecainide and propafenone, detach very slowly from their binding to the channels during diastole. This action eliminates some sodium channels, producing slower conduction; ERP is not prolonged. The duration of the action is unaffected or a slight effect results in minimal slowing of phase 0.
Class II (beta-blockers) inhibit sympathetic stimulation; the main in vitro antiarrhythmic effect of beta-blockers is the depression of phase 4 diastolic depolarization. They are therefore effective in abolishing arrhythmias produced by increased catecholamines. See figure under section Antiarrhythmic Drugs.
Class III (amiodarone, sotalol) cause delayed repolarization, and the duration of the action is prolonged as major action. Amiodarone has class I, II, and III actions. Sotalol has a beta-blocker action and a unique class III effect.
Class IV (verapamil, diltiazem) inhibit slow calcium channel, causing restriction of calcium current.
The experience during the past 20 years indicates that drug actions are much more complex than those given in this classification, but the classification is retained because it has been used worldwide for several years, and it simplifies our understanding of drug action.
Diagnosis of Arrhythmias
The following advice on diagnosis is confined to relevant clinical clues and is of necessity brief. The diagnosis is usually established by careful examination of multiple leads of the electrocardiogram and information derived from carotid massage as appropriate in doubtful cases.
Figure 14-1 gives the differential diagnosis of narrow and wide QRS tachycardias.
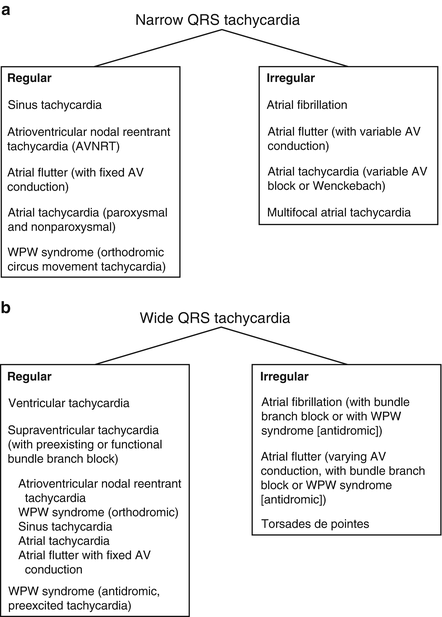

Fig. 14-1.
Differential diagnosis of narrow QRS tachycardia (a) and wide QRS tachycardia (b). Reproduced with permission from Khan MG, Rapid ECG Interpretation, 3rd edition. New York: Humana Press/Springer Science + Business Media; 2008. With kind permission from Springer Science + Business Media.
Arrhythmias with Narrow QRS Complex
1.
Regular
(a)
Ventricular rate 140–240/min: Consider AV nodal reentrant tachycardia (AVNRT) (>70 % of regular SVTs). Retrograde P waves are usually buried within the QRS or at the end of the QRS complex, with a short R–P interval causing pseudo-S waves in II, III, and aVF and pseudo r1 that mimics Rsr1 in V1 . See Fig. 14.2.
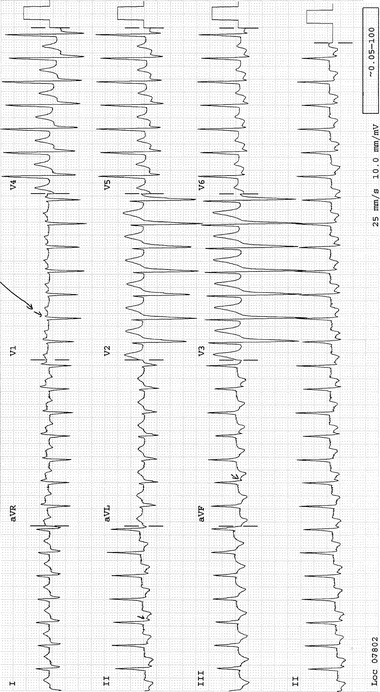

Fig. 14-2.
AV nodal reentrant tachycardia. Note the pseudo-s-wave in lead III (arrow), and pseudo-r1 in V1 (arrow). Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.
(b)
Ventricular rate 100–140/min: Consider atrial tachycardia. P-wave morphology is identical to that during normal sinus rhythm. The arrhythmia accounts for about 5 % of cases of supraventricular tachycardia (SVT).
(c)
Paroxysmal atrial tachycardia (PAT) with block: The P wave is often buried in the preceding T wave.
(d)
Wolff–Parkinson–White (WPW) syndrome with AV reentry (about 15 % of SVTs). See Figs. 14-3 and 14-4.
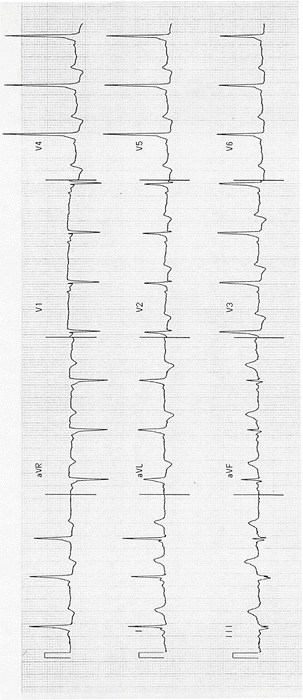
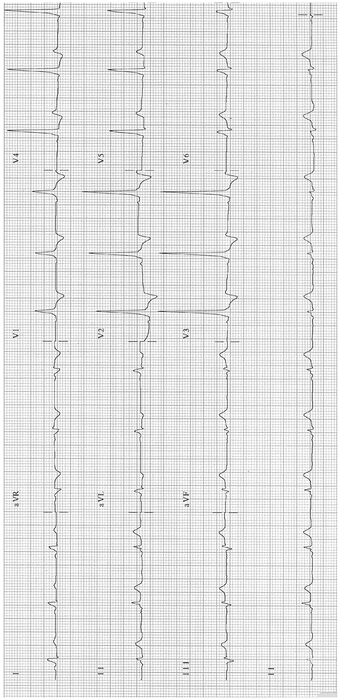

Fig. 14-3.
Sinus rhythm; WPW pattern; short PR and delta wave. Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.

Fig. 14-4.
Tall R-waves V1–V3; type A WPW syndrome. Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.
(e)
Atrial flutter.
(f)
Ectopic atrial tachycardia.
2.
Irregular
(a)
Atrial fibrillation (AF). See Figs. 14-5, 14-6, and 14-7.
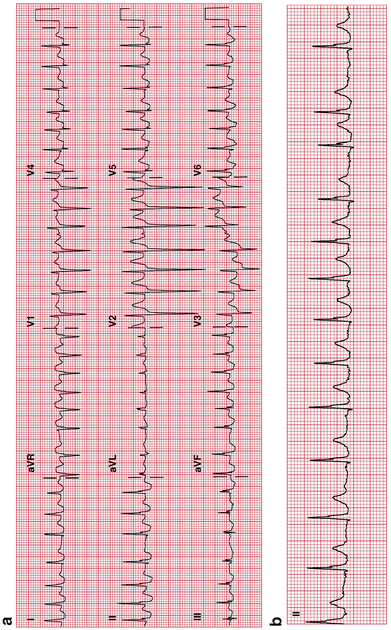
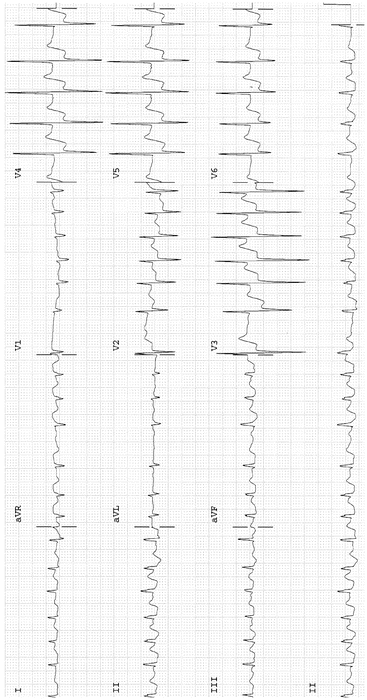
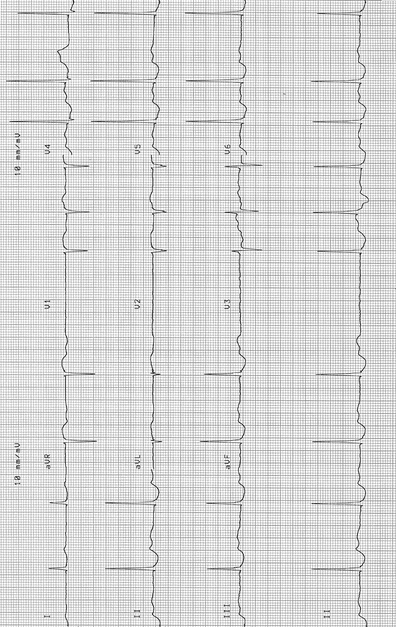

Fig. 14-5.
(a) Atrial fibrillation with a rapid ventricular rate of 175 bpm. (b) Atrial fibrillation, same patient as in (a); controlled ventricular rate of 108 bpm. Lenient study rate control goal 70–109/min (Van Gelder et al. 2010). The European Society of Cardiology (ESC) recommends a resting heart rate target of <110 bpm for heart rate control, the ACCF/AHA/HRS recommends this only if the ejection fraction is >40 %, and the Canadian Cardiovascular Society (CCS) recommends <100 bpm. Reproduced with permission from Khan MG, Rapid ECG Interpretation, 3rd edition. New York: Humana Press/Springer Science + Business Media; 2008. With kind permission from Springer Science + Business Media.

Fig. 14-6.
Atrial fibrillation with rapid ventricular response 152 BPM; marked ST-segment depression in V2–V6, in keeping with subendocardial ischemia, probable non-ST-segment elevation MI. Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.

Fig. 14-7.
Atrial fibrillation, slow ventricular response 60/min: ST–T changes, probable digitalis effect (digoxin level maintained very low at <0.9 ng/ml). This rate should be adjusted to goal rate 70–100/min. The patient is treated with bisoprolol 2.5 mg and digoxin 0.125 mg daily following an episode of heart failure >2 years ago. Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.
(b)
Atrial flutter (when AV conduction is variable). See Figs. 14-8 and 14-9.
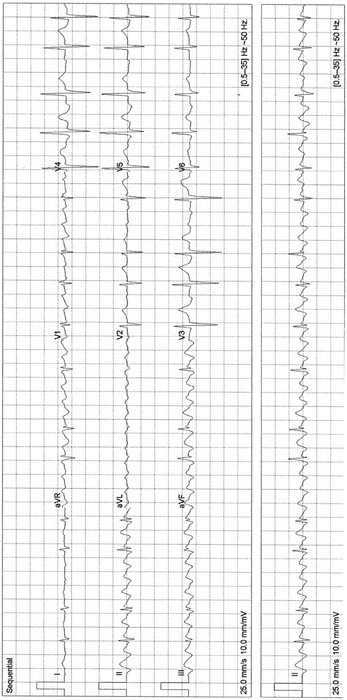
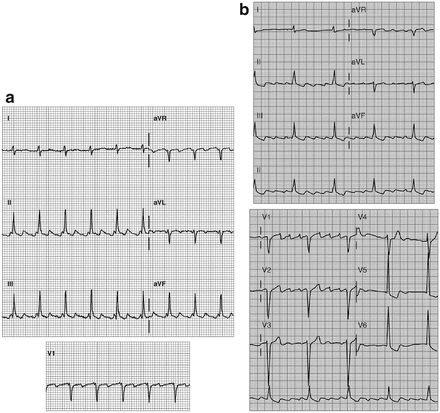

Fig. 14-8.
Atrial flutter; typical flutter waves in leads II, III, and aVF, note the absence of flutter waves in lead I and V6. Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.

Fig. 14-9.
(a) Atrial flutter: atrial rate, 270 BPM; ventricular rate, 135 BPM. Note: The downward deflection of F waves in leads II, III, and aVF has a gradual slope followed by an abrupt upward deflection. This causes the sawtooth pattern. Alternate F waves coincide with the QRS complex, and the diagnosis may be missed. Note: The deformity of the T-waves by the flutter wave revealed in leads II, III, and aVF is absent in lead I, a clue to the presence of atrial flutter. (b) Flutter waves in leads II, III, and aVF; spiky F waves in V1, and notably absence of the sawtooth deformities in lead I, V5, and V6. Reproduced with permission from Khan MG, Rapid ECG Interpretation, 3rd edition. New York: Humana Press/Springer Science + Business Media; 2008. With kind permission from Springer Science + Business Media.
(c)
Multifocal atrial tachycardia (chaotic atrial tachycardia). Varying P-wave morphology, P–P intervals vary, and atrial rate 200–130/min.
Key diagnostic clues for the diagnosis of supraventricular tachycardias are given in Fig. 14-10.
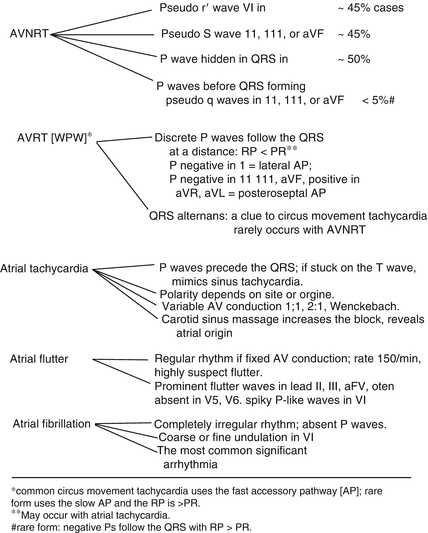

Fig. 14-10.
Supraventricular arrhythmias: key diagnostic clues. (Single asterisk) common circus movement tachycardia uses the fast accessory pathway (AP); rare form uses the slow AP and the RP is > PR. (Double asterisk) may occur with atrial tachycardia. # rare form: negative Ps follow the QRS with RP > PR. Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.
Arrhythmias with Wide QRS Complex
1.
Regular rhythm
A wide QRS complex regular tachycardia is usually caused by VT or SVT with functional aberrant conduction (SVT with preexisting bundle branch block and preexcited tachycardia/SVT with anterograde conduction over an accessory pathway).
Regular wide QRS tachycardia is considered VT if the following clues apply (Steurer et al. 1994):
Predominantly negative QRS complexes in the precordial leads V4–V6: diagnostic for VT (Fig. 14-11).
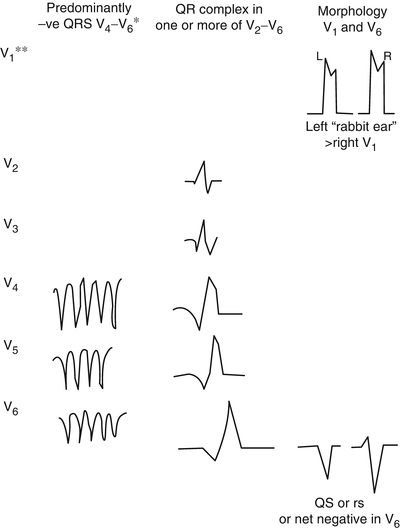
Fig. 14-11.
Electrocardiographic hallmarks of ventricular tachycardia. (Single asterisk) or concordant negativity in leads V1–V6; positive concordance in leads V1–V6 can be caused by ventricular tachycardia or Wolff–Parkinson–White antidromic (preexcited) tachycardia. (Double asterisk) it is necessary to study the entire 12 lead tracing with particular emphasis on leads V1–V6; lead 2 may be useful for assessment of P waves and AV dissociation. Reproduced with permission from Khan MG, Rapid ECG Interpretation, 3rd edition. New York: Humana Press/Springer Science + Business Media; 2008. With kind permission from Springer Science + Business Media.
Presence of a QR complex in one or more of the precordial leads V2–V6: certainly VT.
Absence of an RS complex in all precordial leads: Negative QRS complexes in all precordial leads V1–V6 and negative concordance V1–V6 = VT (Fig. 14-11).
AV relationship different from 1:1: more QRS complexes than P waves; consider VT. Consider SVT with aberrant conduction only in the absence of any of (a) or (b) and with the following present:
QRS morphology similar to intraventricular conduction defect (IVCD) apparent on electrocardiography (ECG) while not in tachycardia.
In basic sinus rhythm.
Visible on atrial ectopic beats = SVT with aberrant conduction. Fixed relationship with P waves, or the presence of a q in V6, suggests but does not prove a supraventricular origin.
Other clues in favor of VT include lead V6: QS or rS; r/S ratio less than 1. Lead V1, V2: If the tachycardia has an LBBB shape and an R wave that is smaller than the r when in sinus rhythm or notched or slurred downslope of the S wave, the left “rabbit-ear” is taller than the right. Figure 14-12 shows salvos of VPBs and ventricular tachycardia.
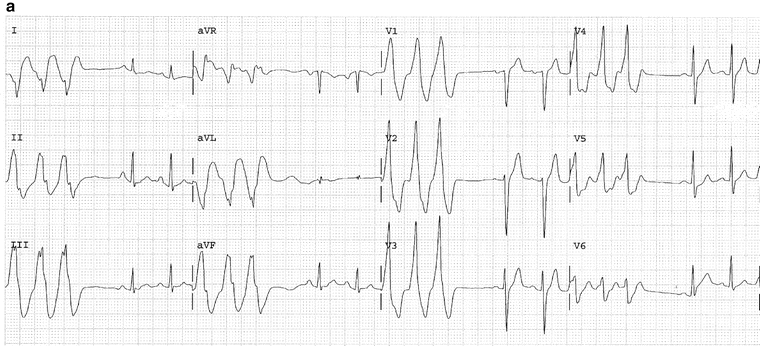
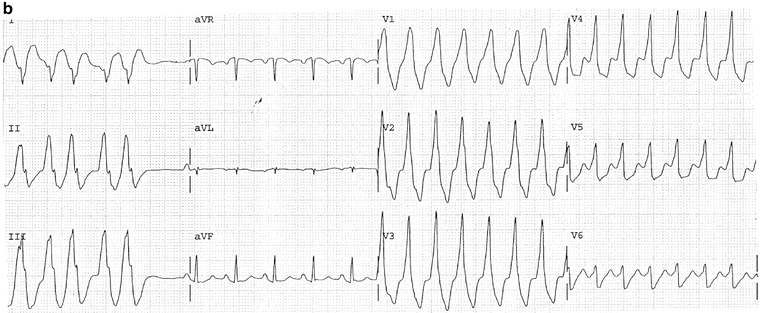
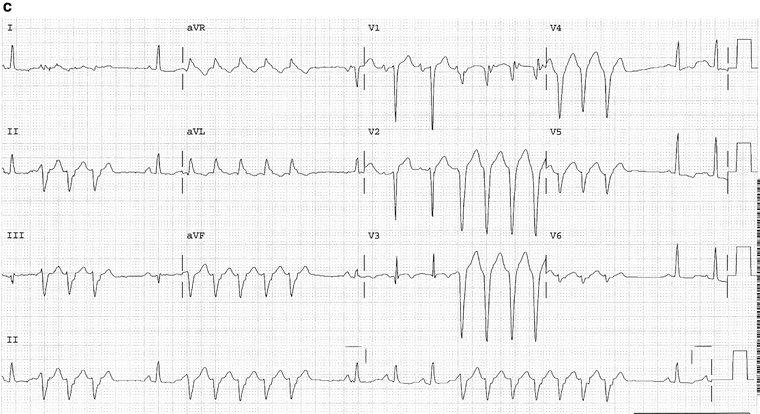
Fig. 14-12.
(a) Salvos of three VPBs. The above trace is (b) The same patient as in Fig. 14a; ECG taken 1 min later shows ventricular tachycardia. (c) Salvos five VPBs, nonsustained VT; complexes in the V leads show negative concordance; typical features diagnostic for VT. Reproduced with permission from Khan MG, Encyclopedia of Heart Diseases, 2nd edition. New York: Springer Science + Business Media; 2011. With kind permission from Springer Science + Business Media.
Figure 14-13 gives ventricular tachycardia: key diagnostic clues.
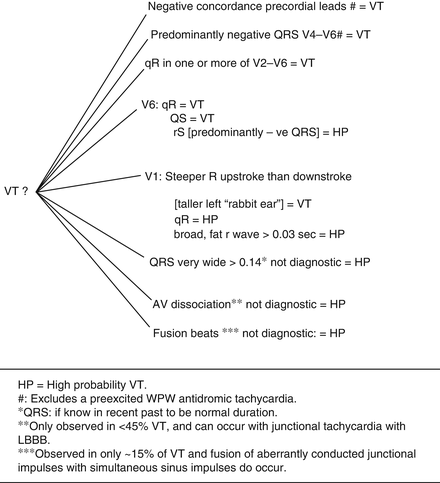
Fig. 14-13.
Ventricular tachycardia: key diagnostic clues. HP = high probability VT. (Number sign) excludes a preexcited WPW antidromic tachycardia. (Single asterisk) QRS: if known in recent past to be normal duration. (Double asterisk) only observed in <45 VT and can occur with junctional tachycardia with LBBB. (Triple asterisk) observed in only ~15 % of VT and fusion of aberrantly conducted junctional impulses with simultaneous sinus impulses do occur. Reproduced with permission from Khan MG, Rapid ECG Interpretation, 3rd edition. New York: Humana Press/Springer Science + Business Media; 2008. With kind permission from Springer Science + Business Media.
Alternative diagnoses to VT or SVT with aberrance:
(a)
Atrial flutter and WPW conduction
(b)
WPW tachycardia (rare type with anterograde conduction, through accessory pathway, preexcited tachycardia)
2.
Irregular rhythm
Atrial fibrillation (AF) and WPW syndrome with a rapid conduction, rate 200–300/min. Figure 14-14 shows AF with preexcited wide QRS tachycardia in a patient with WPW syndrome. Note: The rapidity of the ventricular response (regular or irregular, narrow or wide) should alert the physician to the diagnosis of WPW. AV block or dissociation excludes the presence of a bypass tract:
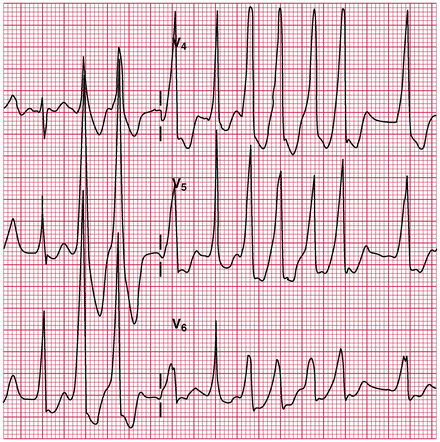

Fig. 14-14.
Atrial fibrillation with preexcited wide QRS tachycardia in a patient with WPW syndrome: antidromic tachycardia. Reproduced with permission from Khan MG, Rapid ECG Interpretation, 3rd edition. New York: Humana Press/Springer Science + Business Media; 2008. With kind permission from Springer Science + Business Media.
AF and IVCD; a previous ECG is needed for comparison.
Management of Supraventricular Arrhythmias
AV Nodal Reentrant Tachycardia
SVT usually arises from reentry mechanisms involving the AV node (AVNRT) and occasionally an accessory pathway, AV reciprocating tachycardia (AVRT), the sinus node, or the atrium. Consequently, drug therapy is directed toward slowing or blocking conduction at some point within the reentry circuit. Whenever the AV node or sinus node is involved directly in the reentry circuit, as is usually the case, SVT is frequently terminated by maneuvers or drugs that increase vagal activity or drugs that slow the velocity of propagation of impulses in the region of the sinoatrial (SA) or AV nodes.
AVNRT in patients aged <35 years usually occurs in an otherwise normal heart, with a good prognosis. It may occur in patients with organic heart disease, e.g., ischemic or rheumatic heart disease, and can be life threatening. The episode is characterized by an abrupt onset and termination. The heart rate varies from 140 to 240/beats/min and the rhythm is regular (Fig. 14-2).
Termination of the Acute Attack of AVNRT
Vagal Maneuvers. Many patients learn to terminate the arrhythmia by gagging or by the Valsalva maneuver (expiration against a closed glottis) or Müller maneuver (sudden inspiration against the closed glottis) or facial immersion in cold water. The Valsalva maneuver is effective in approx 50 % of patients with AVNRT.
Warning. Eyeball pressure has also been used to cause reflex vagal stimulation but is not recommended as retinal detachment may occur.
Carotid sinus massage either causes a reversion to sinus rhythm or has no effect at all. This all-or-none effect is in contrast to the slowing that results when atrial flutter or fibrillation is present. Carotid sinus massage is not recommended in the elderly or in patients with known carotid artery disease. In patients over age 35 years, if a history of carotid disease is suggested by transient ischemic attacks or carotid bruits on auscultation, do not massage the carotid.
The patient must be supine with the head slightly hyperextended and turned a little toward the opposite side. Locate the right carotid sinus at the angle of the jaw. Using the first and second fingers, apply firm pressure in a circular or massage fashion for 2–6 s. Carotid massage is discontinued immediately upon termination of the arrhythmia because prolonged asystole may otherwise supervene in rare patients. If unsuccessful, massage the left carotid sinus.
Warning. Never massage for more than 10 s and do not massage the right and left carotid simultaneously.
In some patients, restoration of sinus rhythm is clearly a matter of great urgency because of hemodynamic deterioration or angina, and direct current (DC) cardioversion is performed either before any drug is given or when the need becomes apparent following unsuccessful drug therapy.
Drug Management in the Acute Situation
1.
Adenosine (Adenocard) 0.05–0.25 mg/kg given IV is an effective and relatively safe agent for the termination of acute reentrant SVT. Adenosine terminates AVNRT and AVRT in up to 90 % of cases (DiMarco et al. 1990; Rankin and McGovern 1991). The 6-mg and 12-mg doses cause a 60–80 % and 90–95 % termination, respectively. The drug has both therapeutic and diagnostic value. The drug has replaced verapamil when very rapid conversion of the arrhythmia is required, as in cases with HF or hemodynamic compromise, thus avoiding DC countershock in many individuals. It can be given to patients with known SVT and aberration; little harm ensues if the diagnosis is in fact VT, whereas verapamil use is detrimental. Nonetheless, the drug is contraindicated in patients with a wide QRS complex tachycardia unless the diagnosis of SVT with aberrancy is certain (Delacrétaz 2006). The drug has no appreciable hypotensive or negative inotropic effect and slows AV nodal conduction.
Dosage: Usually IV bolus 6 mg (0.05–0.25 mg/kg) over 2 s, rapidly flushed into a peripheral vein, followed by a bolus of 20 ml of fluid should cause reversion to sinus rhythm in 1 min; the action of the drug is only about ½ min and, if needed, a 12-mg second bolus injection is given approximately 2 min after the first injection. A further 12-mg dose may be repeated in 3–5 min, as the arrhythmia occurs in 10–33 % of cases. The very short half-life, less than 2 s, allows rapid dose titration to be achieved. Because adenosine may excite atrial and ventricular tissue, it causes atrial fibrillation in up to 12 % of patients and nonsustained VT in rare cases (Strickberger et al. 1997). It should be administered only when a cardiac monitor is being used and a defibrillator is on hand.
Interactions: Dipyridamole potentiates the effects of adenosine. Thus, the dose of adenosine must be reduced in patients taking dipyridamole; significant hypotension may be caused by the combination. Theophylline is an antagonist.
Adverse effects: Transient headache, facial flushing, and dyspnea, but bronchospasm may last more than a half hour in asthmatics. The drug has minor proarrhythmic effects and may cause atrial and ventricular premature beats; rarely SVT or atrial flutter may degenerate to AF (Jaeggi et al. 1999). Atrial flutter is not an indication for adenosine; 1:1 conduction and extreme tachycardia may ensue.
Contraindications: Asthma, severe chronic obstructive lung disease (COPD), and wide QRS complex if the diagnosis of SVT with aberrancy is uncertain and in heart transplant recipients.
2.
Verapamil IV is effective in more than 90 % of episodes of AVNRT (narrow complex).
Contraindications: Verapamil is contraindicated in patients with wide QRS tachycardia, WPW syndrome, severe hypotension, HF, acute MI, known sick sinus syndrome, digitalis toxicity, and concurrent use of beta-blockers or disopyramide.
Dosage: IV 0.075–0.15 mg/kg, i.e., 5–10 mg, is given slowly over 1–2 min. The depressant effect on the AV node may persist for up to 6 h, and a second dose may produce complications. If the arrhythmia persists following Valsalva maneuver or right carotid massage or recurs without hemodynamic deterioration in patients with a normal heart, a second dose not exceeding 5 mg may be considered after 30 min. If restoration of sinus rhythm is clearly urgent, adenosine is tried and then, if needed, DC conversion. IV infusion 1 mg/min to a total of 10 mg or 5–10 mg over 1 h; 100 mg in 24 h. Prolongation of AV conduction induced by verapamil can be reversed by atropine. Calcium gluconate or chloride is useful in the management of hypotension, circulatory collapse, or asystole owing to sinus arrest or AV block. Atropine may be of value in this situation.
3.
Propranolol 1 mg IV given slowly and repeated every 5 min to a maximum of 5 mg; the usual dose required is 2–4 mg. Metoprolol is given in a dose of 5 mg at a rate of 1–2 mg/min repeated after 5 min if necessary to a total dose of 10–15 mg.
4.
Diltiazem IV is as effective as verapamil and causes fewer adverse effects; both agents cost approximately $15 per treatment compared with $100 for two 6-mg doses of adenosine. Diltiazem has a role in patients who have well-defined SVT.
Dosage: Initial bolus 0.25 mg/kg over 2 min and, if needed, rebolus 0.35 mg/kg.
5.
Phenylephrine (Neo-Synephrine) is an alpha-sympathetic agonist. The drug is now rarely used because of better alternatives, especially adenosine. The drug has a role only in young patients with a normal heart, when adenosine has failed, when the blood pressure is <90 mmHg, and when cardioversion is considered undesirable. The resulting increase in blood pressure stimulates the baroreceptor reflexes and increases vagal activity, often resulting in termination of the arrhythmia.
Contraindications: Patients with MI, severe cardiac pathology, and narrow-angle glaucoma.
Dosage: Phenylephrine is administered as repeated IV bolus injections; 0.1 mg is diluted with 5 mL 5 % dextrose and water (D/W) and given over 2 min. Blood pressure is measured at 30-s intervals. Arterial blood pressure must not be allowed to exceed 140 mmHg. Sufficient time (1–2 min) should elapse after each bolus to allow the blood pressure to return to its baseline value before subsequent doses are administered. Dose range: 0.1–0.5 mg. Higher doses have been used but are not recommended. Administration by IV drip infusion may result in variation in drug rates that may cause an unacceptable increase in blood pressure.
Chronic Drug Management of AVNRT
Recurrent prolonged or frequent episodes may require chronic drug therapy. The following may be tried:
1.
Digoxin. This drug is especially useful in patients with left ventricular dysfunction (LV) and in those with resting systolic blood pressure <110 mmHg in whom beta-blockers, verapamil, or diltiazem may cause symptomatic hypotension or bradycardia. Also, the drug is inexpensive and available as a one-a-day tablet. It is not used in patients with WPW syndrome.
2.
A beta-blocker. Choose a once-daily preparation: bisoprolol 5–10 mg daily, metoprolol succinate sustained release (Toprol-XL) 50 mg. Atenolol is not recommended (see Chap. 1) If AVNRT recurs on this regimen, verapamil 80–120 mg orally usually aborts the attack within 1 h and avoids bothersome emergency room visits.
3.
Digoxin plus a beta-blocker may be necessary in patients resistant to (1) and (2).
4.
Verapamil SR 120–180 mg daily or diltiazem 180–240 mg may be tried but is effective in <50 % of patients and is an expensive therapy.
5.
Pill in the pocket. No prophylactic treatment is given. At the onset of an episode, the patient takes verapamil 80–120 mg or a beta-blocker, e.g., metoprolol tartrate (rapid acting) 50 mg or bisoprolol 5 mg orally.
The combination of diltiazem and propranolol terminated 80 % of SVT episodes within 2 h of administration (Alboni et al. 2001).
These regimens are safe and efficacious in patients with normal hearts. Recurrent AVNRT resistant to pharmacologic therapy should prompt consideration of catheter ablation.
Multifocal Atrial Tachycardia (Chaotic Atrial Tachycardia)
This arrhythmia is caused by frequent atrial ectopic depolarizations. The arrhythmia is characterized by variable P-wave morphology and P–P and PR interval. The atrial rate is usually 100–130/min, and the ventricular rhythm is irregular. The diagnosis is made by demonstrating three or more different P-wave morphologies in one lead. The arrhythmia is usually precipitated by acute infections, exacerbation of COPD, electrolyte and acid-base imbalance, theophylline, beta1-stimulants, and, rarely, digitalis toxicity. Digitalis is usually not effective, and treatment of the underlying cause is most important. If the ventricular response is excessively rapid, slowing may be achieved with verapamil given orally. Magnesium sulfate is an effective alternative if verapamil is contraindicated. Adenosine is ineffective.
Paroxysmal Atrial Tachycardia with Block
Episodes are usually associated with severe cardiac or pulmonary disease. PAT is a common manifestation of digitalis toxicity. The atrial rate is commonly 180–220/min. AV conduction is usually 2:1. The rhythm is usually regular. The ventricular rate of 90–120/min may not cause concern, and the P waves are often buried in the preceding T wave, so the diagnosis is easily missed.
If the ventricular rate is 90–120/min and the serum potassium (K+) level is normal, digoxin and diuretics should be discontinued, and often no specific treatment is required. If the serum K+ concentration is <3.5 mEq (mmol)/L and a high degree of AV block is absent, IV potassium chloride 40 mEq (mmol) in 500 mL 5 % D/W is given over 4 h through a central line. If the serum K+ level is <2.5 mEq (mmol)/L, KC1 is best given in normal saline to improve the serum potassium level quickly. Other therapies are outlined under treatment of digitalis toxicity.
Atrial Premature Contractions
Atrial premature contractions (APCs) often occur without apparent cause. Recognized causes include stimulants, drugs, anxiety, hypoxemia, HF, ischemic heart disease, and other cardiac pathology. APCs in themselves require no drug therapy. Treatment of the underlying cause is usually sufficient. In patients with no serious underlying cardiac disease, reassurance is of utmost importance. Stimulants such as caffeine, theophylline, nicotine, nicotinic acid, and other cardiac stimulants as well as alcohol should be avoided.
When heart or pulmonary disease is present, APCs may predict runs of SVT, AF, or atrial flutter, and the resulting increase in heart rate may be distressing to the patient. Digoxin may be useful and, rarely, disopyramide may be necessary. If mitral valve prolapse is associated, sedation or a beta-blocker may be useful.
Atrial Flutter
Underlying heart disease is usually present; however, hypoxemia owing to a pneumothorax, atelectasis, and other noncardiac causes may precipitate the arrhythmia. Atrial flutter tends to be unstable, either degenerating into AF or reverting to sinus rhythm.
The atrial rate is usually 240–340/min. The ventricular rate is often 150/min with an atrial rate of 300, i.e., 2:1 conduction. Therefore a ventricular rate of 150/min with a regular rhythm should alert the clinician to a diagnosis of atrial flutter. The sawtooth pattern in lead II should confirm the diagnosis. See Figs. 14-8 and 14-9.
Carotid sinus massage may increase the degree of AV block, slow the ventricular response, and reveal the sawtooth P waves as opposed to P waves separated by isoelectric segments in PAT with block. Rarely a 1:1 conduction with a rapid ventricular response is seen, especially in patients with preexcitation syndromes or in patients receiving a class I antiarrhythmic agent.
Treatment: Atrial flutter is easily converted to sinus rhythm by synchronized DC shock at low energies of 25–50 J. Electrical cardioversion is often indicated and should be performed if the patient is hemodynamically compromised or if the ventricular response is >200/min or the patient is known or suspected to have WPW syndrome. If the patient is hemodynamically stable with a ventricular response <200/min, propranolol may be used to slow the ventricular response. The benefit of propranolol or metoprolol is that patients who can undergo electrical cardioversion may do so easily, whereas following digoxin DC shocks have been reported to be hazardous. If underlying heart disease is present, digoxin has a role in the acute and chronic management. Digoxin converts atrial flutter to AF, and the ventricular response is nearly always slowed to an acceptable level provided sufficient digoxin is used. Removal of underlying causes may be followed by spontaneous reversion to sinus rhythm. Verapamil or diltiazem is effective in slowing the ventricular response and may occasionally cause conversion to sinus rhythm. Digoxin, verapamil, and beta-blockers are contraindicated in patients with WPW syndrome presenting with atrial flutter. Quinidine, procainamide, or disopyramide must not be used alone for the conversion of atrial flutter to sinus rhythm because these drugs, especially quinidine, increase conduction in the AV node and may result in a 1:1 conduction with a ventricular response exceeding 220/min. If quinidine is administered, it must be preceded by adequate digitalization to produce a sufficient degree of AV block.
Propafenone and flecainide have been shown to convert atrial flutter to sinus rhythm in 20 % and 33 % of patients, respectively.
Atrial Fibrillation
The AHA/ACC provided sound guidelines in 2014. AF is the most common sustained arrhythmia observed in clinical practice. Among patients with a recent cryptogenic stroke or TIA who were 55 years of age or older, noninvasive ambulatory ECG monitoring for a target of 30 days significantly improved the detection of atrial fibrillation by a factor of more than five and nearly doubled the rate of anticoagulant treatment. Atrial fibrillation lasting 30 s or longer was detected in 45 of 280 patients (16.1 %) in the intervention group, as compared with 9 of 277 (3.2 %) in the control group monitored for 24 h (Gladstone et al. 2014).
A prospective study and dose–response meta-analysis indicate that alcohol consumption, even at moderate intakes, is a risk factor for atrial fibrillation (Larsson et al. 2014).
In most patients, drug action to control the ventricular response provides adequate therapy. A ventricular response <60/min in an untreated patient should raise the suspicion of disease of the AV node or concomitant sick sinus syndrome, particularly in the elderly. AF with a slow ventricular rate that becomes regular, indicative of the presence of a junctional pacemaker, should raise the suspicion of digitalis toxicity.
Atrial Fibrillation of Recent Onset
In acute AF, with a fast ventricular rate, especially if there is hemodynamic compromise or with rates exceeding 220/min, WPW syndrome may be the cause, and drugs that block the AV node are contraindicated. Drug therapy to slow the ventricular response in patients with AF, in whom conduction using the accessory pathway can be excluded, includes the following:
1.
Diltiazem IV is the drug of choice for urgent rate control in patients with AF; a constant IV infusion brings the ventricular response under control reliably. Sinus rhythm is achieved in only about 15 %, and hypotension occurs in up to 33 % of patients. Diltiazem IV followed by procainamide IV bolus may cause reversion to sinus rhythm.
2.
Esmolol slows the rate adequately over 20 min, and sinus rhythm may ensue. The drug causes hypotension in up to 40 % of patients. Esmolol and digoxin are effective, and hypotension is much less common than when esmolol alone is used. Digoxin appears to protect from hypotension.
Paroxysmal Atrial Fibrillation
Paroxysmal atrial fibrillation is a major clinical problem that is difficult to manage. The patient should be anticoagulated to prevent embolization. IV disopyramide (not approved in the USA) or procainamide given with digoxin may restore sinus rhythm in <33 %. If the rate <150 is well tolerated, it is advisable to observe the rhythm for 8–12 h because spontaneous rhythm is common, particularly if the patient has taken an extra dose of sotalol (40–80 mg) prior to coming to the emergency room. DC cardioversion should be immediately available if drug therapy fails, when the ventricular rate is rapid, or when hypotension and HF ensue. For the prevention of recurrent episodes, low-dose sotalol 40–60 mg is beneficial in 40–50 % of patients. Although low-dose amiodarone is more effective than sotalol (approx 75 %) and pulmonary side effects run approx 3 %, other side effects may emerge.
Fortunately life-threatening paroxysmal atrial fibrillation is rare. Even severe cases can be rapidly brought under control in the ER, and recurrences in the majority occur after 1–5 years. These patients are anticoagulated with warfarin or preferably rivaroxaban (the latter may prevent MI). Sotalol 80–160 mg or bisoprolol 5–10 mg combined with digoxin to maintain a digoxin level 0.5–0.9 ng/ml (0.6–1.0 nmol/L) should result in a ventricular rate <140/min if a paroxysm occurs patients with stroke with undetermined cause (cryptogenic) may have underlying undetected paroxysmal atrial fibrillation. Monitoring for a target of 30 days significantly improved the detection of atrial fibrillation by a factor of more than five and nearly doubled the rate of anticoagulant treatment, Atrial fibrillation lasting 30 s or longer was detected in 45 of 280 patients (16.1 %) in the intervention group, as compared with 9 of 277 (3.2 %) in the control group monitored for 24 h (Gladstone et al. 2014).
Catheter Ablation
In patients with paroxysmal atrial fibrillation, it is only necessary to rapidly obtain a ventricular rate of <140/min during a paroxysm; a rate 120–150/min is well tolerated by most patients. In patients with left ventricular dysfunction or an ejection fraction of <45 %, a rate >140 may precipitate HF. In such selected patients ablation should be considered. Fortunately this scenario is rare. The rush to ablation should be halted because anticoagulation is still necessary when sinus rhythm is restored and many complications are been reported.
Caution: 83 % of centers reported the use of oral anticoagulants following ablation; death (0.05 %) and stroke (0.28 %) were observed in 6 % of 11,762 procedures (Cappato et al. 2005). This report covered a period during which skill and technologic devices were being developed. Significant PV stenosis and stroke occurred in 1.3 % and 0.2 % of patients, respectively. About half of the PV stenoses required interventional treatment, a strategy that does not necessarily abolish symptoms. Constrictive pericarditis has been reported (Ahsan et al. 2008). Tamponade may occur. Intracranial emboli which typically presents as a transient ischemic attack (TIA) or stroke occurs (Steinberg and Mittal 2011).
A worldwide survey on catheter ablation of AF reported a 0.71 % incidence of TIA and 0.23 % incidence of stroke. Periprocedural complications occurred in 1 of 20 patients undergoing AF ablation, and all-cause and arrhythmia-related rehospitalizations were common (Shah et al. 2012). Among 4,156 patients who underwent an initial AF ablation, 5 % had periprocedural complications, most commonly vascular, and 9 % were readmitted within 30 days. Less hospital experience with AF ablation were associated with higher adjusted risk of complications and/or 30-day readmissions. The rate of all-cause hospitalization was 38.5 % by 1 year. The rate of readmission for recurrent AF, atrial flutter, and/or repeat ablation was 21.7 % by 1 year and 29.6 % by 2 years (Shah et al. 2012). The recurrence rate for atrial fibrillation after the first year is 6–9 % per year (Hussein et al. 2011; Weerasooriya et al. 2011; Ouyang et al. 2010).
Haines emphasized that the real-world success rate of this procedure is unknown but is likely considerably lower than published reports from single-center studies. Also complication rates are significant, as are late hospitalization rates. Data from many investigations in AF ablation and other interventional procedures have demonstrated that operator and hospital procedure volume are inversely correlated with poor outcomes (Haines 2012).
Chronic Atrial Fibrillation
In most patients with chronic AF of more than 1 year’s duration, slowing of the ventricular response will suffice.




A beta-blocker is a good choice even in patients with class I–III HF or asymptomatic LV dysfunction.< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


