ESSENTIALS
Some drugs, e.g. beta-blockers, anti-arrhythmics and anti-cholinergics in particular, are important causes of arrhythmias.
- Triggered activity – occurs as a result of membrane voltage instability. Ionic disturbances, specifically calcium and sodium ions, may alter the membrane potential of cardiac myocytes resulting in abnormal depolarisations (early or delayed) occurring after an action potential. This is known as afterdepolarisations. Long QT syndrome and ventricular arrhythmias may arise due to this mechanism.
2. Altered conduction
- Conduction block occurs when the conducting wave of depolarisation is terminated as it is blocked by unexcitable myocardial tissue. There are two ways in which this can happen.
- fixed block – the wave of conduction is inhibited by a physical barrier i.e. scarred tissue
- functional block – the conducting impulse encounters refractory tissue, preventing further wave propagation. This is often caused by drugs that prolong the action potential of myocytes (class III anti-arrhythmics e.g. amiodarone)
- fixed block – the wave of conduction is inhibited by a physical barrier i.e. scarred tissue
- Re-entry occurs when the conducting impulse travels around an abnormal re-entrant loop circuit. In order for re-entry to happen, there must be two distinct conduction pathways with different conduction velocities. This can occur as a large anatomical circuit as seen in Wolff–Parkinson–White syndrome via the normal conduction system and the accessory pathway, or as smaller circuits in the AV node and spiral micro-circuits seen in atrial fibrillation. The mechanism of re-entry is shown in Figure 11.1.
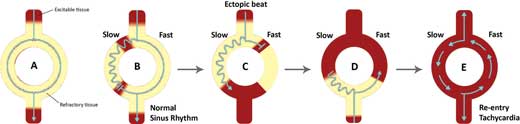
Figure 11.1 – Mechanism of re-entry.
A. In a normal heart, the wave of depolarisation travels down both pathways at similar velocities. They eventually meet and the resultant impulse is propagated distally.
B. When there are two pathways with different conduction velocities, the time taken for the wave of depolarisation to travel down the slow pathway is longer than that of the fast pathway. This may allow retrograde transmission of impulses of the fast pathway up the slow pathway. However, when this retrograde impulse encounters refractory tissue of the slow pathway, both conduction waves are terminated. As a result, only impulses travelling down the fast pathway are propagated distally. This process is known as unidirectional block.
C. When an ectopic beat reaches the circuit while the fast pathway is still in its refractory period, the wave of depolarisation is only allowed to travel down the slow pathway.
// Why? //
The refractory period of the slow pathway is shorter than that of the fast pathway. This is a fundamental feature in the mechanism of re-entry.
D. As the impulse reaches the distal end of the circuit, the fast pathway has repolarised, allowing retrograde propagation of the impulse. Re-entry has now occurred.
E. The conduction wave may then loop around the circuit, initiating a tachycardia as the impulse is propagated distally after each loop. This re-entrant circuit may continue indefinitely until it is interrupted by a change in electrical depolarisation.
Investigations
A 12-lead electrocardiogram should be performed in all patients with suspected arrhythmias (refer to Chapter 4). Further investigations may be useful in confirming the diagnosis and identifying the underlying cause.
- Blood test: FBC (anaemia may cause a sinus tachycardia), U&Es (hyperkalaemia and hypercalcaemia may predispose to arrhythmias), TFTs (hyperthyroidism is an important cause of atrial fibrillation and other tachyarrhythmias)
- Ambulatory 24-hour Holter recording: may be useful in patients with frequent but transient episodes
- Echocardiography: performed when there is evidence of ischaemic or structural heart disease
- Electrophysiology (EP) studies: performed in two groups of patients:
- patients with paroxysmal episodes suitable for ablation therapy
- high-risk patients with disabling symptoms and evidence of ischaemic heart disease.
- patients with paroxysmal episodes suitable for ablation therapy
Therapeutic modalities
The management of patients with arrhythmias can be fairly complex. Pharmacological modalities, particularly anti-arrhythmic drugs, were once the mainstay treatment for arrhythmias but their use has declined since the advent of percutaneous interventional electrophysiological procedures. However, anti-arrhythmic drugs are still used in clinical practice, mainly as an adjunct to other therapies. Pharmacological therapy of arrhythmias has been discussed in Chapter 5.
Interventional electrophysiology
Electrical therapy for arrhythmias involves delivering low-voltage electrical impulses to stimulate cardiac myocyte depolarisation, particularly in the case of bradycardias (pacing) or higher voltage shocks to globally and transiently depolarise the heart, resetting its rhythm, in particular during life-threatening tachyarrhythmias (cardioversion).
Pacing. There are two forms of pacing that are usually performed for the treatment of bradycardias.
- Temporary pacing is used in the acute setting to stabilise patients who are haemodynamically unstable.
// PRO-TIP //
Temporary pacing can be performed in one of two ways:
- Trans-thoracic external pacemaker
- delivered through large gel electrodes over the chest wall
- rapid administration may be uncomfortable, as high currents are required to penetrate the thoracic wall
- delivered through large gel electrodes over the chest wall
- Trans-venous pacing
- percutaneous catheter insertion via the internal jugular, subclavian or femoral vein into the heart. The pacing wire is usually placed in the right ventricle.
- more complex procedure but can be used for longer periods (few days)
- percutaneous catheter insertion via the internal jugular, subclavian or femoral vein into the heart. The pacing wire is usually placed in the right ventricle.
- Permanent pacemaker (PPM)
- sub-dermal implantation of a pulse generator and a battery, most commonly inferior to the clavicle as well as placement of pacing electrodes either in the right atrium or right ventricle (single chamber) or both (dual chamber)
- possesses ‘sense’ and ‘pace’ functions that detect the cardiac rhythm and only delivers electrical pulses when necessary
- common complications include lead displacement, wound infection and pneumothorax.
- sub-dermal implantation of a pulse generator and a battery, most commonly inferior to the clavicle as well as placement of pacing electrodes either in the right atrium or right ventricle (single chamber) or both (dual chamber)
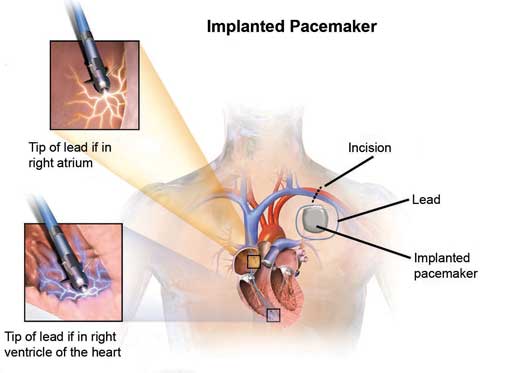
Figure 11.2 – Dual chamber permanent pacemaker and the location of its pacing electrodes.
Cardioversion (also known as defibrillation). The aim of cardioversion is to restore normal sinus rhythm. Electrical cardioversion involves the delivery of a high voltage direct current (DC) biphasic shock to completely depolarise the heart, terminating the tachyarrhythmia.
// Why? //
A biphasic shock is used to minimise the total amount of shock delivered to the patient. The polarity of the shock is reversed (i.e. positive to negative or vice versa) halfway through. This effectively halves the energy needed.
EXAM | In patients with organised ventricular rhythms (e.g. VT) or patients with AF or flutter, it is important to synchronise the shock with the early part of the QRS complex. This is because delivery of a shock during the T wave on the surface ECG might result in initiation of ventricular fibrillation. |
Cardioversion is primarily used in the treatment of ventricular arrhythmias, atrial fibrillation or atrial flutter. There are two types of cardioversion, electrical and chemical (pharmacological) cardioversion.
- Electrical (external) cardioversion – also known as DC cardioversion
- Chemical (internal) cardioversion – involves the use of intravenous anti-arrhythmic drugs (e.g. amiodarone, procainamide) and may be indicated in patients with AF and atrial flutter.
Implantable cardioverter defibrillators (ICDs)
- ICDs are very similar to permanent pacemakers and as such, they detect life-threatening tachyarrhythmias and deliver electrical shocks to terminate them
- It is important to note that in addition to their defibrillation function, ICDs also have pacing functions
- They are used in both primary and secondary prevention of sudden cardiac death from ventricular arrhythmias (see below).
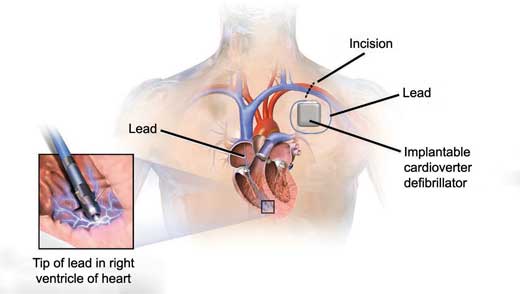
Figure 11.3 – Implantable cardioverter defibrillator.
GUIDELINES: Implantable cardioverter defibrillators for arrhythmias (NICE, 2014)
NICE recommends ICDs as primary prevention for patients with:
• Strong family history of a heart condition with a high risk of sudden death (e.g. long QT syndrome, Brugada syndrome, etc.)
• Congenital heart disease that has been repaired surgically.
NICE also recommends secondary prevention for patients who:
• Survived a cardiac arrest caused by a ventricular arrhythmia
• Have spontaneous sustained VT causing syncope or severe haemodynamic instability
• Have sustained VT without syncope and a LVEF of less than 35%.
Radiofrequency catheter ablation
This technique involves the utilisation of radiofrequency waves to heat and ablate the focus/foci of enhanced automaticity or re-entrant circuits causing tachyarrhythmias. EP studies are usually performed prior to the procedure to localise the abnormal tissue.
Catheter ablation has become the definitive treatment for patients with SVTs, in particular atrioventricular nodal re-entrant, atrioventricular re-entrant tachycardias and atrial flutter. Cure rates of over 90% are expected in such patients.
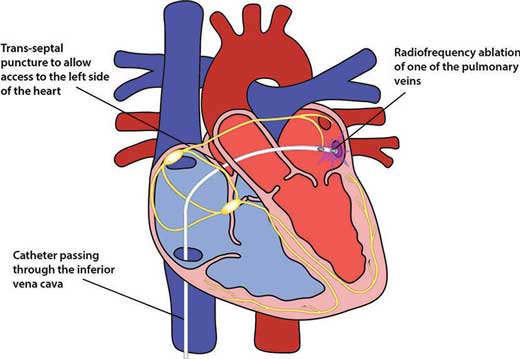
Figure 11.4 – Radiofrequency ablation.
11.2 Systematic approach to ECG rhythm abnormalities
As discussed in Chapter 4, an ECG can be interpreted in a few easy steps. Similarly, arrhythmias can be systematically approached with the following algorithm:
1. First, take a look at the heart rate – determine whether it is a bradycardia (<60 bpm) or a tachycardia (>100 bpm)
2. If it is a bradycardia,
- and asymptomatic – it will most likely be sinus bradycardia but heart block should be ruled out
- and symptomatic/haemodynamically unstable – atrioventricular (AV) block should be suspected and managed appropriately
3. If it is a tachycardia, determine whether it is a narrow complex (QRS <0.12 s) or a broad complex (QRS >0.12 s)
- Narrow complex tachycardias are supraventricular in origin i.e. SVT and can be distinguished from one another based on their specific ECG patterns (e.g. saw tooth in atrial flutter)
- All broad complex tachycardias should be considered ventricular in origin (VT or VF) until proven otherwise as these patients are commonly haemodynamically unstable. It is important to treat broad complex tachycardia as VT because treatments for SVT could make a patient with VT become haemodynamically unstable.
- An SVT with aberrant conduction (e.g. atrial fibrillation with a bundle branch block) can also present with a broad complex tachycardia appearance. As a very general rule of thumb:
- Regular rhythms are likely to be VT
- Irregular rhythms are more likely to be AF with aberrant conduction
4. Check to see if there are any Extra Beats or Missing Beats
Extra beats | Missing beats |
|
|
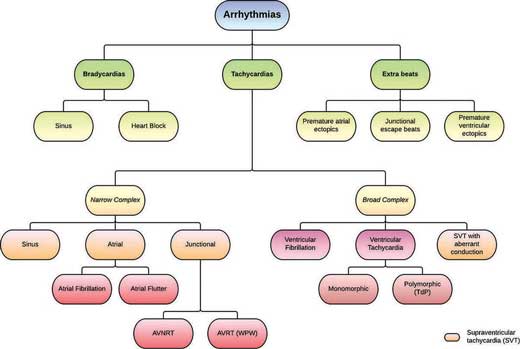
Figure 11.5 – Schematic representation of arrhythmias.
11.3 Bradycardias
Bradyarrhythmias are disorders of slow heart rhythm. They occur as a result of reduced impulse production (e.g. sinus bradycardia) or failure of impulse propagation due to failure of the conducting system (e.g. atrioventricular blocks or bundle branch blocks). Each of these conditions will be discussed further in the sections below.
11.3.1 Sinus bradycardia
Definition
A decrease in the heart rate to less than 60 beats per minute in adults. Normal sinus rhythm is observed.
Epidemiology
- Affects 20–25% of under 25 year olds.
Aetiology
Common causes:
- Physiological – during sleep, athletes, young people
- Cardiac – inferior myocardial infarction, sick sinus syndrome (see below)
// Why? //
The SA nodal artery is a branch of the right coronary artery (RCA) in 90% of the population. In an inferior myocardial infarction, the RCA could be involved, and blood flow to the SA node is also affected, leading to bradycardia.
- Drugs – beta-blockers, calcium channel blockers, digoxin, opiates.
Other causes:
- Metabolic – hypothyroidism, hyperkalaemia, hypothermia
- Neurological – brain stem pathology (e.g. raised intracranial pressure, infarction)
// PRO-TIP //
Increased intracranial pressure (from a haemorrhage or hydrocephalus) causes an increase in blood pressure to maintain cerebral perfusion. However, aortic baroreceptors are stimulated in response to the increased blood pressure and in turn, trigger a parasympathetic response. In addition, vagal compression as a result of the increased intracranial pressure further activates this response and ultimately induces bradycardia. This is known as the ‘Cushing’s reflex’. This is a sign of impending brain herniation and death.
Pathophysiology
In intrinsic SA node disease, the automaticity of the node is depressed due to ageing or any disease that affects the atrium such as ischaemic heart disease and cardiomyopathy. On the other hand, extrinsic factors such as drugs and metabolic imbalances suppress SA node activity by reducing its automaticity via vagal activation.
Clinical features
- Symptoms range from fatigue to syncope.
ECG appearance

Figure 11.6 – An ECG showing an RR interval of 2.8 s (14 large boxes; 0.2 s) equivalent to a rate of 21 bpm.
Management
- Treatment not usually required for asymptomatic patients
- Treat underlying cause – stop offending medication
- Atropine may be indicated in acute setting in patients with severe bradycardia
- If patients are unresponsive to atropine, consider isoprenaline and temporary pacing.
11.3.2 Sick sinus syndrome
Definition
A syndrome of SA nodal dysfunction that encompasses sinus bradycardia, sinus pause and sinoatrial block.
EXAM | Sick sinus syndrome is often associated with other SVTs, in particular atrial fibrillation and atrial flutter. When the atrial arrhythmia terminates, there is a prolonged sinus node recovery time, which can result in syncope. This combination is termed ‘tachycardiac-bradycardia syndrome’. |
Epidemiology
- More common in elderly patients
- Seen in 0.2% of patients over the age of 50
Aetiology
Common causes:
- Idiopathic fibrosis of the SA node (as a result of ageing)
- Myocardial ischaemia
Other causes:
- Infiltrative conditions – sarcoidosis, amyloidosis
- Drugs – beta-blockers, digoxin, calcium channel blockers, amiodarone
- Metabolic – hypothyroidism, hyperkalaemia
- Cardiomyopathies.
Pathophysiology
Degenerative and ischaemic changes in the SA node, nerve supply, and the surrounding atrial tissue comprise the underlying pathology of sick sinus syndrome. This will either result in abnormalities of impulse formation, impulse conduction or both. The failure to produce a sinus impulse, i.e. sinus arrest or pause, occurs as a result of reduced automaticity. In addition, fibrosis of the SA node and atrial myocytes may also result in conduction blocks (commonly presenting as sinoatrial block).
// PRO-TIP //
Fibrosis of atrial tissue contributes to the arrhythmogenicity of atrial fibrillation and atrial flutter.
Clinical features
- Chronic frequent episodes of intermittent bradycardia and tachycardia
- Palpitations
- Dizziness or syncope.
ECG appearance
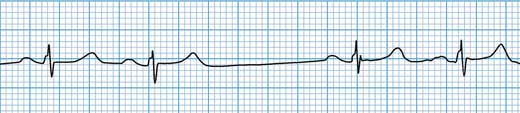
Figure 11.7 – Sinus node exit block. There is a missing P wave. The sinus node has ‘fired’ but the impulse has failed to propagate into the atrium.

Figure 11.8 – A long sinus pause (>3 s) is seen as a result of SA node failure; the subsequent beat is a junctional escape.
Management
- Treat underlying cause if present
- Intravenous atropine may be useful in patients with severe symptoms
- Dual chamber pacemaker implantation recommended for patients with symptomatic chronic disease.
GUIDELINES: Dual-chamber pacemakers in sick sinus syndrome (NICE, 2014)
NICE recommends dual-chamber pacemaker implantation for all patients with symptomatic bradycardia due to sick sinus syndrome with or without the presence of an atrioventricular conduction block.
11.4 Atrioventricular conduction blocks
Atrioventricular (AV) conduction blocks refer to a disturbance in impulse conduction between the atria and ventricles. This can be permanent or transient depending on the aetiology of the block. Disturbances at different sites within the AV conduction system (AV node and the His-Purkinje system) produce AV blocks of varying severity. Classically, AV blocks have been split into three categories:
11.4.1 First-degree heart block
Definition
Delayed atrioventricular conduction resulting in a constant prolonged PR interval (>0.2 s) on ECG.
Epidemiology
- Commonly affects patients over the age of 65.
Aetiology
Common causes:
- Idiopathic degeneration (fibrosis) of the conduction system
- Increased vagal tone (e.g. athletes, during sleep)
- Myocardial ischaemia (RCA supplies the AV node)
- Drugs (beta-blockers, calcium channel blockers, digoxin)
Other causes:
- Myocarditis
- Metabolic disturbances (hypokalaemia, hypomagnesaemia)
Pathophysiology
First-degree heart block tends to involve the AV node itself. Structural causes such as fibrosis or damage to the AV nodal inputs will delay impulse conduction. Furthermore, the AV node is richly innervated by the autonomic nervous system. Therefore, vagal (parasympathetic) activation may also prolong AV conduction time.
Clinical features
- Usually asymptomatic.
ECG appearance
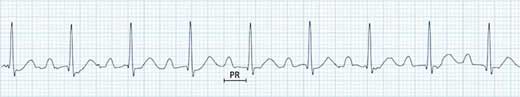
Figure 11.9 – ECG with a prolonged PR interval (240 ms) but otherwise normal.
- PR interval (>0.2 s or 5 small squares)
- Sinus rhythm (each P wave is followed by a QRS complex).
Management
- Benign condition; treatment is not usually required.
Prognosis
- Normal, although some patients will progress to higher degrees of AV block over time.
11.4.2 | Second-degree heart block: Mobitz type I (also known as the Wenckebach block) |
In second-degree heart block, there is intermittent failure of atrioventricular conduction resulting in occasional dropped beats. There are two forms of second-degree heart block, Mobitz Type I and Mobitz Type II. The mechanism and pathophysiology of both types are quite dissimilar and so are their clinical presentation and findings.
Definition
An atrioventricular conduction deficit resulting in progressive prolongation of the PR interval until a beat is dropped.
Epidemiology
- Occurs in 4% of post-inferior MI
- More common than Mobitz type II.
Aetiology
Common causes:
- Idiopathic fibrosis of the conduction system
- Drugs (beta-blockers, calcium channel blockers, digoxin, procainamide)
- Increased vagal tone – athletes, children, during sleep
Other causes:
- Iatrogenic – transcatheter aortic valve implantation (TAVI)
- Inferior MI
- Other causes are similar to those of first-degree heart block.
Pathophysiology
Mobitz type I heart block is caused by progressive conduction block, more commonly within the AV node itself (70%) and sometimes more distally (30%) in the conduction system. Mobitz type I can also be vagally mediated as a result of normal physiology or drugs and rarely caused by structural abnormalities. Mobitz type I differs from first-degree heart block in that there is progressive AV nodal cell fatigue, eventually resulting in a dropped beat.
Clinical features
- Majority of patients are asymptomatic
- May present with light-headedness, dizziness and syncope or exertional fatigue.
ECG appearance
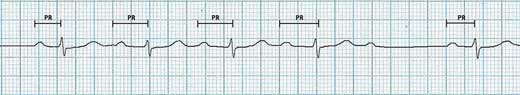
Figure 11.10 – Wenckebach phenomenon – prolongation of four preceding PR intervals before the 5th beat is dropped.
- Progressive prolongation of the PR interval until a beat is dropped
- Narrow QRS complexes
- PR interval longest before the dropped beat and shortest after.
Management
- Treatment not usually required unless symptomatic
- IV atropine may be used in emergency situations or in very severe bradycardia
- Permanent pacemaker implantation is indicated in patients with non-resolving symptomatic block. This is shown to have mortality benefits in patients above the age of 45.
11.4.3 | Second-degree heart block: Mobitz type II – non-Wenckebach block |
Definition
An atrioventricular conduction deficit resulting in intermittent dropped beats without changes in the PR interval.
Aetiology
Common causes:
- Idiopathic fibrosis of the conduction system
- Anterior MI
// Why? //
An anteroseptal myocardial infarction may damage the His-Purkinje system as the conduction bundle lies within the septum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


