■ An arterial phase CT angiogram should evaluate the entire length of the aorta, from the level of the skull base proximally to the femoral heads distally, to ensure visualization of the vertebral and iliofemoral arteries, respectively. The CT images are then processed using 3D imaging software for case planning and device selection. A magnetic resonance imaging (MRI) or a noncontrast CT scan will not suffice.
■ A transthoracic (2D) echocardiogram should be performed to assess left and right ventricular function and to exclude the presence of significant valvular heart disease.
■ Strong consideration should be given to evaluating the anatomy of the coronary arteries in the preoperative period. A CT coronary angiogram may be an option for younger patients or those with complex proximal aortic dissection. However, if there is a strong suspicion of coronary disease, then a preoperative conventional coronary angiogram should be performed, including those patients older than 40 years of age and those with a history of smoking.
SURGICAL MANAGEMENT
Preoperative Planning
■ Indications for repair of an aortic arch aneurysm include large aneurysmal size (>5.5 cm), rapid growth (>0.5 cm per year), the presence of chest pain or back pain unexplained by other causes, and compression of adjacent organs (esophagus, trachea, or left main bronchus).2
■ More aggressive size criteria may be applied for patients with Marfan’s syndrome (repair at 4.5 to 5 cm). However, stent graft outcomes appear less favorable in patients with connective tissue disease, and therefore, alternative surgical techniques (such as conventional aortic replacement surgery) should be considered.2
■ The presence of significant concurrent cardiac disease may alter the surgical approach. Should significant coronary artery or valvular heart disease be identified in the preoperative period, consideration may be given to performing concomitant coronary artery bypass grafting (CABG) or valve replacement at the time of the aortic debranching procedure.
■ During the second stage of the arch repair, stent graft deployment in the distal ascending aorta may require the placement of a guidewire across the aortic valve into the left ventricular cavity. The presence of a mechanical aortic prosthetic valve, through which a guidewire and the delivery system cannot safely be placed, may require a single-stage approach with deployment of the stent graft at the time of debranching (see endovascular second stage). A bioprosthetic valve in the aortic position may allow for careful transvalvular introduction of devices, with preference to bovine pericardial valves over porcine valves.
■ Selection of the ideal treatment strategy for repair of an aortic arch aneurysm remains controversial and is dictated by surgical experience and local area expertise. Aortic arch debranching and stent graft completion is an appealing repair option that avoids a thoracotomy incision and may avert the use of cardiopulmonary bypass and circulatory arrest. These types of hybrid procedures may be performed either as single- or two-stage repairs. However, conventional open replacement of the entire aortic arch,3,4 or replacement of the ascending aorta and proximal arch with the creation of an elephant trunk followed by stent graft completion,1,5 should be considered as clinically indicated.
■ Debranching of the aortic arch off the ascending aorta may not be applicable for a patient with an aortic arch aneurysm who has previously undergone cardiac surgery and who is too high-risk for consideration of redo sternotomy. In this case, an alternative option would include extra-anatomic debranching of the aortic arch (carotid–carotid, carotid–subclavian) followed by stent graft repair of the arch, with or without innominate artery chimney (snorkel) stenting.6
■ The preoperative CT scan requires careful review before undertaking an aortic arch debranching operation. Arch branch anatomy and appropriate landing zones need to be identified proximal and distal to the arch aneurysm, with criteria similar to those that apply for stent graft repair of a descending thoracic aortic aneurysm. Anatomic variations of the aortic arch anatomy may require modification of the debranching procedure. These include a bovine aortic arch (common trunk of the innominate and left common carotid), arch origin of left vertebral artery, and an aberrant right subclavian artery.
■ The ascending aorta is typically 6 to 7 cm in length from the sinotubular junction to the innominate artery. Placement of the proximal inflow anastomosis as low as possible on the ascending aorta (just distal to the sinotubular junction) will result in an optimal 3- to 4-cm proximal landing zone for the stent graft repair. The largest currently available thoracic stent grafts are 42 to 46 mm in diameter. To provide a safe and durable proximal landing zone and avoid a proximal type I endoleak, we recommend replacement of an ascending aorta that is extremely short or if its diameter is 36 mm or larger. Open replacement of the ascending aorta would be performed at the time of the arch debranching procedure, with implantation of an aortic graft 34 mm or smaller.
■ The size of the iliofemoral arteries is worth noting on the preoperative CT study. The external iliac arteries need to be larger than 7 mm in diameter to provide adequate vascular access to deliver the stent graft devices during the second stage. An iliac artery conduit may be needed if the iliofemoral arteries are extremely small or in the presence of severe calcification and occlusive disease. Alternatively, a single-stage antegrade introduction of the stent graft from the ascending aorta may be performed (see endovascular second stage) to avoid access problems from a retrograde iliofemoral approach.
■ The diameters of the brachiocephalic arteries are measured on the preoperative CT scan to determine the interposition graft sizes for the debranching procedure. Most frequently, the size of the graft chosen for the innominate artery branch is 10 to 14 mm, with 6- to 8-mm grafts usually used for the left carotid and left subclavian arteries.
■ Cerebral oximetry monitoring may be helpful for the aortic debranching procedure to monitor brain perfusion before and after clamping of the brachiocephalic arteries. For the second-stage endovascular procedure, cerebrospinal fluid (CSF) drains are placed preoperatively to reduce the risk of spinal cord ischemia if a significant length of the descending thoracic aorta is to be covered.
Positioning
■ For the arch debranching procedure, patients are positioned supine just as they are during standard cardiac surgical operations. Prepping is performed from the neck to the knees, with draping higher than usual to strategically provide access to the lower neck. The head may be turned slightly to the right to facilitate extension of the sternotomy incision proximally along the left sternocleidomastoid muscle.
TECHNIQUES
AORTIC ARCH DEBRANCHING
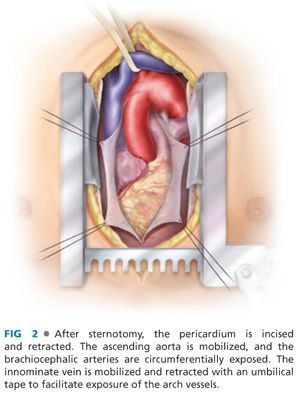
■ Although some advocate the use of a right thoracotomy incision or upper hemisternotomy, we prefer to expose the ascending aorta through a conventional sternotomy incision. This provides optimal visualization and control. The pericardium is incised and retracted.
■ The ascending aorta is carefully mobilized to facilitate later placement of a proximally positioned side-biting clamp. The space between the left side of the aorta and the pulmonary artery is dissected, with small vessels cauterized or clipped and divided. The ascending aorta is mobilized proximally down to the level of the aortic root (sinotubular junction) to enable identification (and avoid injury) to the right coronary artery.
■ The brachiocephalic arteries are circumferentially exposed. The innominate vein is mobilized and retracted with an umbilical tape to facilitate exposure of the arch vessels (FIG 2). Uncommonly, the innominate vein requires ligation and division to aid in arch exposure. The left subclavian artery is often more posterior than expected, and exposure of this artery may be difficult. In these circumstances, the sternotomy incision may be extended superiorly and leftward along the sternocleidomastoid muscle. Alternatively, innominate and left carotid debranching may be combined with a left carotid-subclavian bypass/transposition procedure, through a standard supraclavicular approach, obviating the need to expose the left subclavian artery through the sternotomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


