Approach to the Patient with Complicated Myocardial Infarction
Richard C. Becker
Acute coronary syndromes (ACS) are best defined as atherothrombotic disorders of the coronary arterial system. Although the underlying pathoanatomic substrate exhibits features of chronicity developing slowly over decades, the defining events of plaque disruption, intraluminal thrombosis, and distal embolization are acute and occur suddenly, often without warning. For this reason, clinicians involved directly in the management of patients with ACS must be well versed in a broad range of diagnostic and treatment strategies to rapidly provide the highest possible level of care.
Acute myocardia infarction (MI) is not infrequently complicated by potentially lethal events that can be categorized as vascular (recurrent coronary thrombosis, cardioembolism, or venous thromboembolism), myocardial or mechanical (ventricular dilation, aneurysm formation, pump failure, ventricular septal rupture, papillary muscle rupture, free-wall rupture), pericardial (pericarditis), electrical (heart block, bradyarrhyth-mias, tachyarrhythmias) and metabolic (Table 14-1 and Fig. 14-1). Most complications occur within the initial 72 hours of infarction; however, the early risk period extends to include the first 4 to 6 weeks. Pharmacologic treatments, including antithrombotic agents and fibrinolytics, can also cause complications that require prompt management. Critical pathways, which represent a readily available and implementable means to facilitate consistently high levels of care among patients with complicated MI, are highlighted in this chapter.
Setting the Stage for Complications
Fundamental Concepts
Acute MI, caused most often by coronary arterial thrombosis that impairs myocardial blood flow and tissue perfusion and less commonly as a result of excessive myocardial oxygen demand, is defined pathologically as an irreversible change or death of an individual cell (myocyte) or, in most cases, group of cells. The necrotic process can be identified approximately 6 hours from the onset of myocardial hypoperfusion and is characterized initially by a heavy infiltration of neutrophils that persists for approximately 48 hours. Within 7 days of infarction, the myocardium thins as necrotic tissue is steadily removed by mononuclear cells and phagocytes. Granulation tissue infiltrates the involved region 1 week later and, in essence, covers the entire area by 3 to 4 weeks. Over time (typically within 12 to 16 weeks), the zone of infarction contracts to become a thin, white, firm scar (1,2,3,4).
Cellular Characteristics of Wound Healing
The infiltration of inflammatory cells, an immediate response to tissue injury in acute MI, includes neutrophils, lymphocytes, macrophages, and fibroblasts. Collagen degradation, also an early response to myocardial necrosis, involves matrix metalloproteases (MMP) that reside within the myocardium in latent forms (5,6,7,8). The abrupt release of inflammatory mediators and MMP contribute to coronary arterial thrombosis and plaque instability, respectively, providing, at least in part, one
explanation for the heightened propensity for thromboembolic events that persists following an initial event. These same me-diators may also play a role in cellular apoptosis (programmed myocyte death). The inflammatory infiltrate, coupled with a significant degree of surrounding edema, creates a profound effect on electrical conduction and refractory periods. Beyond the “irritability” of necrotic myocardium that can cause automatic ventricular arrhythmias, the differing characteristics of injured and healthy myocardium existing “side-by-side” create “dispersion of refractoriness”—the substrate for re-entrant ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation. Lastly, collagenase activity within the myocardium, although designed to permit “rebuilding” of the damaged area, may initially weaken the infarct zone, increasing the risk of cardiac rupture.
explanation for the heightened propensity for thromboembolic events that persists following an initial event. These same me-diators may also play a role in cellular apoptosis (programmed myocyte death). The inflammatory infiltrate, coupled with a significant degree of surrounding edema, creates a profound effect on electrical conduction and refractory periods. Beyond the “irritability” of necrotic myocardium that can cause automatic ventricular arrhythmias, the differing characteristics of injured and healthy myocardium existing “side-by-side” create “dispersion of refractoriness”—the substrate for re-entrant ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation. Lastly, collagenase activity within the myocardium, although designed to permit “rebuilding” of the damaged area, may initially weaken the infarct zone, increasing the risk of cardiac rupture.
Table 14-1. Complications of Acute Myocardial Infarction | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
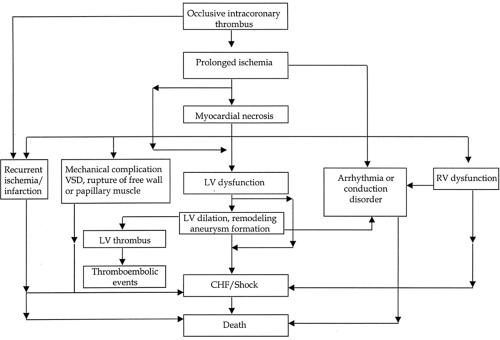 Figure 14-1. Pathobiologic and clinical sequence of events in myocardial infarction. CHF, congestive heart failure; LV, left ventricular; RV, right ventricular; VSD, ventricular septal defect. |
The fibrous stage of wound healing follows an initial inflammatory stage. Increased synthesis of fibrillar type III collagen and, over the subsequent days to weeks, type I collagen, provides an organized “scaffold” for scar development that follows. It is during this stage that most myocardial remodeling takes place, including expansion of both the infarct- and noninfarct-related zones, leading to aneurysm formation and ventricular dilation.
In the final stage, fibrillar fibronectin and collagen are deposited and by 4 to 6 weeks most of the necrotic myocardium has been removed and replaced by fibrous scar tissue. As with the initial or inflammatory stage, the late scarring stage is characterized by marked heterogeneity of repolarization and refractory periods, creating a permissive environment for malignant arrhythmias.
Early Risk Characterization
A vital component of clinical practice and critical care medicine is an ability to anticipate complications either of the MI itself or of its treatment. The development and utilization of risk-assessment scales facilitates a response to potentially life-threatening events and represents the logical “first” step in patient-specific management pathways.
Identification of Patients at High Risk
Early Clinical Phase
Risk stratification schemes for patients with ST-segment elevation MI (STEMI) have been developed by several experienced clinical trial groups, highlighting the importance of patient demographics, medical history, clinical features, and the presenting electrocardiogram (ECG). The Thrombolysis in Myocardial Infarction (TIMI) study investigators (9) established a risk score that predicted, with considerable accuracy, the occurrence of early morbidity and mortality.
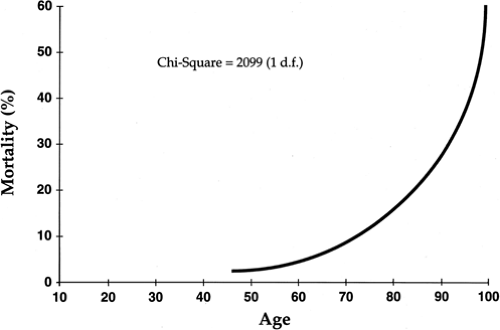
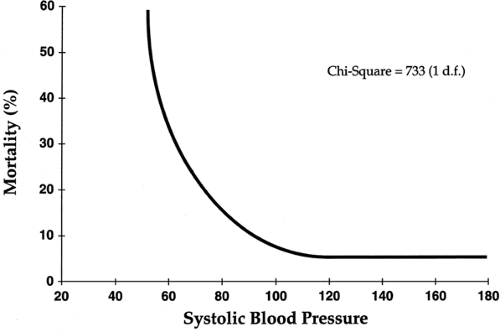
Predictors of early (30-day) mortality were also established by the GUSTO (Global Utilization of Streptokinase and tPA for Occluded Coronary Arteries) investigators (10). In a trial including more than 40,000 patients with acute MI, age was identified as the most significant factor, with death rates of 1.1% in the youngest decile (<45 years) and 20.5% in patients more than 75 years of age. Overall, five characteristics contained 90% of the prognostic information in the baseline clinical data—they included age, lower systolic blood pressure, higher Killip class, elevated heart rate, and anterior site of infarction (Figs. 14-2 and 14-3).
The National Registry of Myocardial Infarction (NRMI) risk assessment assignment was developed as a readily available, bedside clinical “scorecard” for rapid triage and management. Predictors of adverse in-hospital clinical outcomes were identified from more than 100,000 patients with MI (11).
Presenting Surface Electrocardiogram
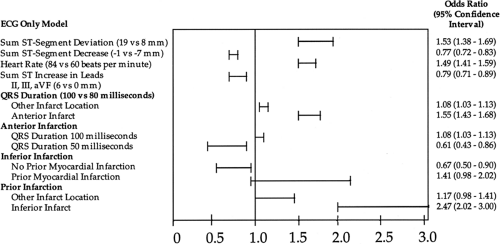
Clinicians have recognized for some time that the sum of ST-segment “shifts” on the presenting ECG is a reliable marker of infarction size and, as a result, can provide important diagnostic (and prognostic) information. The initial ECG may also contain evidence of a prior MI, identifying patients at increased risk for an early adverse outcome (Fig. 14-4) (12). ST-segment area also provides prognostic information.
Cardiac Enzyme Analysis
Biochemical markers of myocardial necrosis approximate infarction size and, in general, provide an estimate of clinical
outcome. The alteration of pharmacokinetics, particularly for creatine kinase (CK), following coronary reperfusion has complicated the picture somewhat. In essence, when calculated correctly, the “area under the curve” does provide prognostic information, but this measurement requires time (serial CK determinations) and effort.
outcome. The alteration of pharmacokinetics, particularly for creatine kinase (CK), following coronary reperfusion has complicated the picture somewhat. In essence, when calculated correctly, the “area under the curve” does provide prognostic information, but this measurement requires time (serial CK determinations) and effort.
The prognostic value of cardiac-specific troponin T and I among patients with unstable angina and non–ST-segment elevation MI is recognized; however, data from the Global Use of Strategies To open Occluded Arteries (GUSTO) IIA trial (13) also suggest that an elevated troponin T (>0.1 ng/mL) on hospital presentation correlates strongly and independently with hospital mortality among patients with STEMI.
Inflammatory Markers
The measurement of inflammatory markers represents an important tool that links the triad of atherosclerosis, inflamma-tion, and thrombosis. Recent observations with the acute phase reactant amyloid A protein in all likelihood will open the door to a vast array of markers that ultimately can be used to determine the “activity” of disease and direct response to treatment (14).
Risk for Stroke
Stroke is the most feared and devastating complication of fibrinolytic therapy (15). Overall, 60% of patients with hemorrhagic
stroke die, whereas 25% are left with at least a moderate degree of disability (Table 14-2).
stroke die, whereas 25% are left with at least a moderate degree of disability (Table 14-2).
Table 14-2. Risk Factors and Predictors of Hemorrhagic Stroke Following Thrombolysis: Gusto-1 Trial | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Non–ST-Segment Elevation MI
The medical community has placed considerable emphasis on the early management of patients with STEMI or bundle branch block MI because of the profound impact of early reperfusion on patient outcome. It has become clear that patients with non–ST-segment elevation MI also require careful consideration, not solely because of their recognized risk for cardiac events over the subsequent 6 to 12 months, but also because a substantial number of patients are at risk for in-hospital death.
A total of 183,113 patients with non–ST-segment elevation MI were identified in NRMI-2 (16). Risk factors for in-hospital death included advanced age (odds ratio [OR] 1.51), female sex (OR 1.20), history of diabetes (OR 1.22), prior congestive heart failure (OR 1.30), and Killip class II, III, and IV (OR 1.61, 1.94, and 21.4, respectively) (Table 14-3).
Table 14-3. Predictors of In-Hospital Death (After 24 H)a | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Recurrent Ischemia and Infarction
Recurrent Ischemia
The two most common cardiac causes of recurrent chest pain among hospitalized patients with acute MI are acute pericarditis and myocardial ischemia; the latter occurs more often and is a marker of poor outcome. Recurrent myocardial ischemia occurs in 15% to 20% of patients and is typically transient (17). It does, however, identify patients with a persistently unstable atherosclerotic plaque and heightened thrombotic potential, as well as those with multivessel coronary artery disease or compromised collateral circulation (18).
Patients with recurrent ischemia can be further stratified according to ECG changes, hemodynamic stability, and overall clinical status. Three distinct categories of patients have been identified as being at increased risk for postinfarction angina and reinfarction: (a) patients with non–ST-segment elevation MI, (b) patients receiving fibrinolytic therapy, and (c) patients with multiple cardiac risk factors (19). The incidence of postinfarction angina is nearly twice as high after non–ST-segment elevation MI (25% to 35%) than after ST-segment elevation or bundle branch block MI (15% to 20%). Patients treated with fibrinolytics have a 20% to 30% incidence of recurrent ischemia and a 4% to 5% incidence of reinfarction, even with the concomitant use of aspirin and adjunctive anticoagulant strategies (20).
Management
Recurrent ischemia must be recognized promptly and managed aggressively. Pain relief is best approached in the context of myocardial oxygen supply and demand. Based on this fundamental construct, myocardial oxygen demand can be lowered by decreasing heart rate, inotropic state (using intravenous or oral beta-blockers), and preload (most commonly with nitrate preparations). Morphine sulfate, in low doses, is also useful in early management. A calcium channel blocker can be added, if needed, to control heart rate, to prevent episodic coronary vasospasm, or as an alternative to beta-blocker therapy when absolute contraindications exist. Antiplatelet (aspirin ± clopidogrel) therapy should be continued and anticoagulation with intravenous unfractionated heparin or low-molecular-weight heparin (LMWH) instituted (or continued) for patients with ischemic chest pain at rest. In addition, consideration should be given to the addition of a GP IIb/IIIa antagonist. Anxiolytics such as benzodiazepines can also be used, as needed, to reduce anxiety and provide mild sedation. The goals of therapy are to reduce mean arterial blood pressure and heart rate by approximately 10% to 20%, but not to a level where coronary arterial perfusion pressure is compromised. In clinically unstable patients with evidence of congestive heart failure, volume status must be assessed carefully and treated appropriately.
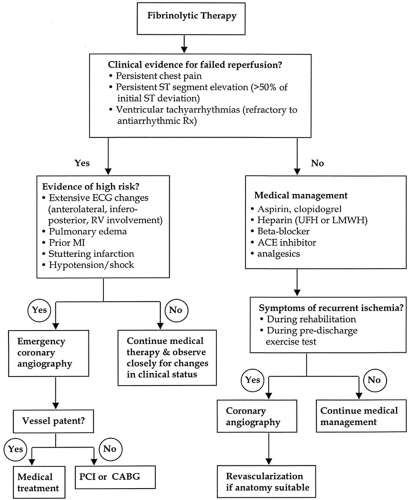
When aggressive pharmacologic therapy does not alleviate the myocardial ischemia or if concomitant hemodynamic instability exists, early diagnostic coronary angiography is recommended. Consideration should also be given to inserting an intra-aortic balloon pump to improve myocardial perfusion and serve as a “bridge” to definitive therapy (revascularization, correction of mechanical defects) in hemodynamically unstable patients and those with angina refractory to medical therapy
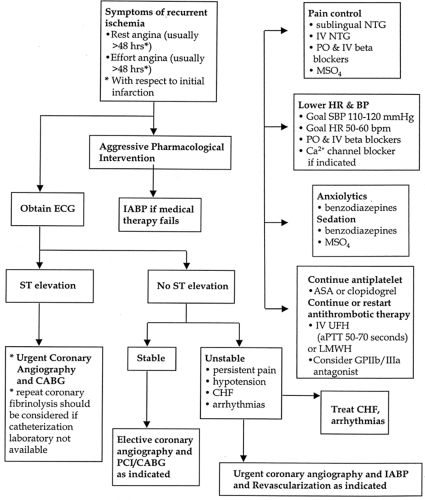
Serial ECGs and clinical assessment are recommended to guide optimal management (Fig. 14-5).
Serial ECGs and clinical assessment are recommended to guide optimal management (Fig. 14-5).
Myocardial Reinfarction
Reinfarction represents a recurrent atherothrombotic event with subsequent myocardial necrosis. The diagnosis can be difficult to confirm within the initial 24 hours of the index event because serum cardiac markers have not yet returned to a normal range. Thus, confirmation must be established in the context of an existing elevation with further (usually twofold) elevation of cardiac enzymes above a prior level (determined within the previous 6 hours). Beta-adrenergic blockers have been shown to reduce the risk of reinfarction, whereas fibrino-lytic therapy is associated with a slightly increased risk (21,22). Heart-rate-slowing calcium channel blockers may reduce the rate of reinfarction among patients with preserved left ventricular (LV) function after acute MI, whereas the role of unfractionated heparin is controversial (23). In contrast, the available data strongly support the ability of LMWH and GP IIb/IIIa receptor antagonists to reduce the likelihood of recurrent MI in patients with non–ST-segment elevation MI and possibly in STEMI or bundle branch block MI as well (24,25).
Management
The clinical approach to recurrent MI, as with recurrent myocardial ischemia, is determined by the patient’s overall clinical status and early indicators of injury (Fig. 14-6).
Coronary Angiography and Percutaneous Coronary Interventions
Early recurrent myocardial ischemia, persistent ST-segment elevation (>50% of maximal ST-segment deviation), hemodynamic instability, and ventricular tachyarrhythmias refractory to antiarrhythmic therapy are indications for early coronary angiography (26). The American College of Cardiology/American Heart Association Guidelines for Coronary Angiography and Percutaneous Coronary Intervention are outlined in Table 14-4.
Surgical Revascularization
Although the procedure is typically reserved for a carefully selected patient, outcomes following emergent surgical revascularization have improved over time as a result of increasing experience; the application of mechanical, hemodynamic, or circulatory support measures; and advanced methods of myocardial protection and anesthetic management (Table 14-5).
Surgical intervention for mechanical complications of MI and cardiac transplantation will be discussed in a subsequent section.
Myocardial and Mechanical Complications
Right Ventricular Infarction
Right ventricular (RV) infarction encompasses a complex spectrum of pathologic and clinical presentations, ranging from asymptomatic mild RV dysfunction to overt cardiogenic shock. RV infarction complicates 35% to 50% of inferior MIs, which in turn make up 40% to 50% of all acute MIs. The diagnosis of RV extension is important because of its association with a higher mortality rate (25% to 30%) (27). Only a small percentage (∼5%) of patients with acute MI present with isolated RV infarction.
Table 14-4. Early Invasive Evaluation in Patients with Stemi | |||||||
|---|---|---|---|---|---|---|---|
|
The clinical triad of hypotension, clear lung fields, and increased jugular venous pressure in a patient experiencing an inferior MI strongly supports the diagnosis of RV extension. A right-sided S3 gallop or Kussmaul’s sign (distention of the jugular veins during inspiration) may also be present on physical examination. Other potential clinical features of RV infarction include tricuspid regurgitation, sinoatrial (SA) or atrioventricular (AV) nodal conduction disturbances, and AV dissociation. The ECG reveals ST-segment elevation of more than 0.5 mm in V4R. A Q wave in this lead is considered a nonspecific finding. Other available modalities that can be used to diagnose RV infarction include thallium or sestamibi perfusion imaging, echocardiography, cardiac magnetic resonance imaging (MRI), and through hemodynamic measurements obtained using a pulmonary artery catheter.
Complications of acute RV infarction, in most instances, are a manifestation of both LV and RV dysfunction, as well as increased parasympathetic tone (Table 14-6). Overt cardiogenic shock, although occurring rarely, is the most serious complication. High-degree AV block is not uncommon and identifies a patient at particularly high risk. Atrial fibrillation occurs in one-third of patients with RV infarction as a result of concomitant right atrial infarction or dilation caused by volume and pressure overload. Other potential complications of RV infarction include ventricular septal rupture (particularly in patients with concomitant transmural posterior septal infarction), RV thrombus formation with subsequent pulmonary embolism, tricuspid regurgitation, and a high incidence of pericarditis, most likely because of the frequent transmural injury pattern of the thin-walled RV. The development of a right-to-left shunt through a patent foramen ovale is a complication unique to RV infarction and should be suspected when severe hypoxia is not responsive to supplemental oxygen therapy (28).
Management
As with all ST-segment elevation MI, the initial approach to patients with RV infarction must address the need for early reperfusion therapy directed at limiting infarct size (Fig. 14-7). Fibrinolytic therapy and primary angioplasty, when successful, improve RV ejection fraction and reduce the incidence of complete heart block. If hemodynamic compromise is present, measures should be implemented to maintain RV preload, reduce RV afterload, and support the dysfunctional RV with pharmacologic inotropic agents. The requirement for volume (preload-dependent state) differentiates the treatment of RV infarction
from that of “pure” LV infarction. Volume expansion is the mainstay of therapy, with the goal of maintaining a right atrial or central venous pressure between 12 and 15 mm Hg. Normal saline (250 to 500 mL) given as a bolus should be used acutely, with an appreciation that a large volume of fluid may be required to sufficiently increase RV filling pressure, LV preload, and cardiac output. If volume support does not produce hemodynamic improvement, a pulmonary artery catheter may be required to guide further management.
from that of “pure” LV infarction. Volume expansion is the mainstay of therapy, with the goal of maintaining a right atrial or central venous pressure between 12 and 15 mm Hg. Normal saline (250 to 500 mL) given as a bolus should be used acutely, with an appreciation that a large volume of fluid may be required to sufficiently increase RV filling pressure, LV preload, and cardiac output. If volume support does not produce hemodynamic improvement, a pulmonary artery catheter may be required to guide further management.
Table 14-5. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Table 14-6. Potential Complications of Right Ventricular Infarction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Patients with persistent hypotension (despite volume resuscitation) may benefit from inotropic support with either dobutamine or dopamine. Because of their potential to reduce preload, vasodilators, including nitroglycerin and morphine sulfate, routinely used in the management of LV infarctions, should be used with great caution in patients with RV infarction. Another crucial factor in sustaining adequate preload is the maintenance of AV synchrony. In patients with high-degree AV block, dual-chamber pacing may be required, particularly if ventricular pacing does not cause an improvement in the patient’s overall clinical status (29). Atrial fibrillation can cause profound hemodynamic deterioration, necessitating prompt cardioversion. In patients with biventricular failure, circulatory support using intra-aortic balloon counterpulsation may be required followed by coronary angiography and either percutaneous
or surgical intervention as the findings and clinical status dictate (Fig. 14-8).
or surgical intervention as the findings and clinical status dictate (Fig. 14-8).
Left Ventricular Dysfunction
The extent of LV compromise, which correlates directly with the extent of myocardial damage, is a major determinant of clinical outcome (Fig. 14-9). Anterior site of infarction is commonly associated with extensive LV damage, reduced ventricular performance, and reduced survival. The Killip classification separates patients into four distinct groups based on existing clinical signs and symptoms of LV failure. Increasing Killip class, which represents progressively severe LV compromise, is associated strongly with a poor prognosis (Table 14-7).
The immediate functional consequences of acute myocardial ischemia and infarction include both systolic and diastolic ventricular dysfunction; either can compromise ventricular performance and lead to congestive heart failure. Diastolic dysfunction (impaired ventricular relaxation) occurs uniformly in patients with acute MI, but is clinically evident in only 20% to 30% of patients. When it does occur, diastolic dysfunction often precedes systolic dysfunction and is the most common cause of early congestive heart failure. Systolic dysfunction, also known as pump failure (typically forward and backward failure), is a serious complication of acute MI. The sudden loss of contractile function decreases stroke volume and increases end-systolic volume, end-diastolic volume, and diastolic filling pressure. The clinical manifestations of systolic dysfunction include decreased forward flow (perfusion) and increased backward flow (pulmonary congestion and edema).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree