Approach to the Management of Thoracic and Thoracoabdominal Aneurysms
Shadi Abu-Halimah
Mark A. Farber
An aneurysm is currently defined as a localized dilatation of the aorta, 50% over the normal diameter, which includes all three layers of the vessel, intima, media, and adventitia. Thoracic aortic aneurysms are less common than aneurysms of the abdominal aorta.
The normal size for the thoracic and thoracoabdominal aorta is larger than that of the infrarenal aorta. The average diameter of the mid-descending thoracic aorta is 26 to 28 mm, compared with 20 to 23 mm at the level of the celiac axis.
Diagnosing and treating thoracic aneurysms (TAs) or thoracoabdominal aneurysms (TAAAs) have evolved in last few decades, but recent advances in endovascular surgery and adjuncts of open surgery potentially alter the prognosis of these aneurysms. However, TAAA repair is still associated with significant mortality and morbidity.
CLASSIFICATION
There are two major subtypes of aneurysm morphology: fusiform, which is uniform in shape with symmetrical dilatation that involves the entire circumference of the aortic wall, and saccular, which is more localized and appears as an out pouching of only a portion of the aortic wall. A pseudoaneurysm or false aneurysm is a collection of blood and connective tissue outside the aortic wall, usually the result of a contained rupture.
Aneurysms of the thoracic aorta can be classified into four general anatomic categories, although some aneurysms involve more than one segment:
Ascending aortic aneurysms arise anywhere from the aortic valve to the innominate artery—60%
Aortic arch aneurysms include any TA that involves the brachiocephalic vessels—10%
Descending aortic aneurysms distal to the left subclavian artery—40%
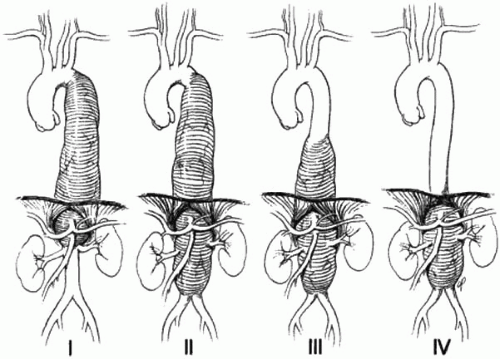
Thoracoabdominal aneurysms—10% TAAAs can also be divided according to the Crawford classification, which was originally designed to help stratify patients into risk categories based upon the extent of disease:
I. Proximal descending thoracic to proximal abdominal aorta
II. Proximal descending to infrarenal aorta
III. Distal descending with abdominal aorta
IV. Primarily abdominal aorta
EPIDEMIOLOGY
The incidence of thoracic aortic aneurysm is estimated to be six to ten cases per 100,000 patient-years.
TAs occur most commonly in the sixth and seventh decade of life.
Males are affected approximately two to four times more commonly than females.
Hypertension is an important risk factor, being present in over 60% of patients.
Associated aneurysms can be detected in approximately 13 % of patients with TAs. Approximately one forth of patients with a large thoracic aortic aneurysm also has an abdominal aortic aneurysm (AAA).
The Crawford classification (Fig. 13.1) is based on the extent of aortic involvement. Extent I aneurysms begin above the sixth intercostal space, usually near the left subclavian artery, and extend down to encompass the aorta at the origins of the celiac axis and superior mesenteric arteries; although the renal arteries may also be involved, the aneurysm does not extend into the infrarenal segment. Extent II aneurysms also arise above the sixth intercostal space but extend distally into the infrarenal aorta, often to the level of the aortic bifurcation. In some cases, they may also involve the ascending aorta. Extent III aneurysms begin in the distal half of the descending thoracic aorta, below the sixth intercostal space, and extend into the abdominal aorta. Extent IV aneurysms generally involve the entire abdominal aorta from the level of the diaphragm to the bifurcation.
ETIOLOGY AND PATHOGENESIS
The true etiology of aortic aneurysms is probably multifactorial, and the condition occurs in individuals with multiple risk factors. Risk factors include smoking, chronic obstructive pulmonary disease (COPD), hypertension, atherosclerosis, male gender, older age, high BMI, bicuspid or unicuspid aortic valves, genetic disorders, and family history. Aortic aneurysms are more common in men than in women and are more common in persons with COPD than in those without lung disease. TA most often results from cystic medial degeneration that leads to weakening of the aortic wall, which is highly associated with atherosclerosis in almost 80% of the cases. Cystic medial degeneration occurs normally with aging and is increased with hypertension.
In patients younger than 60 years of age, the discovery of a TA implies a genetic disorder. This may be the result of Ehlers-Danlos, Marfan’s syndrome, and LD syndrome. Other causes may include inflammatory/infective cases.
Matrix metalloproteinases (MMPs) constitute a family of zinc-dependent proteases (endopeptidases) whose catalytic action is the degradation of the extracellular matrix components. In addition, they play the major role in the degradation of collagen and in the process of tissue remodeling. Multiple investigators have looked specifically at the profiles of proteolytic enzymes in the aortic wall of aneurysm patients and compared these profiles to those of normal individuals. These have implicated the MMP enzymes in the wall of the aorta as a prime agent of deleterious change in aneurysm and dissection disease. They found a marked elevation of the proteolytic enzymes (MMPs) and a marked depression of the inhibitory enzymes (TIMPs). Thus, in aneurysm patients, the balance is shifted strongly toward increased proteolysis—indicating an enzymatic attack on the fibrillin and collagen that form the structural basis of the aortic wall.
Association with Atherosclerosis
More than 80% of the descending TAs are associated with atherosclerosis sharing the same risk factors (e.g., hypertension, hypercholesterolemia, and smoking). It seems likely that there is a multifactorial, systemic, nonatherosclerotic causal process, such as a defect in vascular structural proteins, with atherosclerosis occurring secondarily.
Most theories emphasize the primary role of breakdown of the extracellular matrix proteins, elastin, and collagen by proteases such as collagenase, elastase, various MMPs, and plasmin. These proteolytic factors are derived from endothelial and smooth muscle cells and from inflammatory cells infiltrating the media and adventitia.
The combination of protein degradation and mechanical factors are thought to cause cystic medial necrosis, which has the appearance of smooth muscle cell necrosis and elastic fiber degeneration with cystic spaces in the media filled with mucoid material. These changes result in vessel dilatation and subsequent aneurysm formation and possible rupture.
The following observations in animal models are consistent with the importance of plasmin and metalloproteinases in aortic aneurysm formation.
Blockade of plasmin formation by overexpression of plasminogen activator inhibitor-1 prevents the formation of aneurysms and rupture by inhibiting metalloproteinase activation.
Aneurysm rupture correlates with an increase in metalloproteinase (gelatinases A and B) levels; local overexpression of tissue inhibitor of MMPs, produced by retrovirally infected smooth muscle cells, can prevent aneurysmal degeneration and rupture.
Genetic Factors
Connective tissue disorders such as the Marfan’s or Ehlers-Danlos syndromes are associated with TA and TAAA. But familial associations with TAs have been reported in some patients without these disorders in 19% of TAs and thoracic dissection cases. Mutations in FBN1 have been identified in some patients with ascending thoracic aortic aneurysms who do not have Marfan’s syndrome. Mutations in the transforming growth factor beta receptor 2 gene are responsible for about 5% of familial cases.
Marfan’s Syndrome
Marfan’s syndrome is associated with aortic root dilatation (normal <35 mm) due to cystic medial degeneration prior to aneurysm formation. This disorder is due to mutations in the fibrillin-1 (FBN1) gene.
Aortic root disease, which leads to the formation of aneurysmal dilatation, aortic regurgitation, and dissection, is the main cause of morbidity and mortality in Marfan’s syndrome. Involvement of other segments of the thoracic aorta, the abdominal aorta, or even the carotid and intracranial arteries is reported.
Dilatation of the aorta is found in 50% of children and will progress with time. Echocardiography demonstrates that 60 to 80% of adult patients have dilatation of the aortic root (normal diameter <35 mm), often with aortic regurgitation. Marfan’s syndrome is also frequently associated with aortic dissection, which typically begins just above the coronary ostia, that is, type A dissection, but can extend distally.
Ehlers-Danlos Syndrome Type IV
Etiology of this syndrome is due to defects in type III collagen that causes hyperelasticity and fragility of the skin and hypermobility of the joints. Although aortic root dilatation is uncommon, spontaneous rupture of large and medium-sized arteries, usually without dissection, is reported and is the most serious complication.
Loey-Dietz Syndrome
Loey-Dietz syndrome is a recently discovered autosomal dominant genetic syndrome, which has many features similar to Marfan’s syndrome, but which is caused by mutations in the genes encoding transforming growth factor beta receptor 1 (TGFBR1) or 2 (TGFBR2).
Bicuspid Aortic Valve and Aortic Coarctation
There is an association between bicuspid aortic valve and ascending thoracic aortic aneurysms. In a study of young men with normally functioning bicuspid aortic valves, enlargement of the aortic root and/or ascending aorta was noted in 52%; this finding was independent of hemodynamic abnormalities, age, or body size. The tendency to aneurysm formation is associated with cystic medial degeneration and with decreased expression of FBN1, the gene for which is mutated in Marfan’s syndrome.
In addition, a bicuspid aortic valve is an independent predictor of ascending aortic aneurysm formation after surgical correction of aortic coarctation and is associated with aortic root dilatation in patients with Turner’s syndrome.
Inflammatory/Infectious Disorders
Aortitis has been associated with variety of inflammatory and infectious diseases, which can lead to aortic aneurysm. These diseases include giant cell arteritis, syphilitic aortitis, mycotic aneurysm often due to bacterial endocarditis, Takayasu arteritis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, reactive arthritis, Wegener’s granulomatosis, and reactive arthritis. TA formation is a particular problem in patients with giant cell arteritis that occurs in as many as 11% of patients and may be associated with aortic dissection. Chest x-ray is a recommended screening even for asymptomatic patients.
CLINICAL PRESENTATION
Patients with TAs are often asymptomatic at the time of presentation. Pain can be the presenting symptom according to the location of the aneurysm. This is usually attributed to compression or distortion of adjacent structures or vessels. Aortic regurgitation and thromboembolic disease can be the primary manifestation but is less common.
Ascending aneurysms are more prone to present with heart failure due to aortic regurgitation from aortic root dilatation and annular distortion. Compression of a coronary artery can result in myocardial ischemia or infarction, while a sinus of Valsalva aneurysm can rupture into the right side of the heart, producing a continuous murmur and sometimes heart failure.
Ascending and arch aneurysms can erode into the mediastinum compressing its structures: hoarseness due to compression of left vagus or left recurrent laryngeal nerve; hemidiaphragmatic paralysis due to compression of the phrenic nerve; wheezing, cough, hemoptysis, dyspnea, or pneumonitis if there is compression of the tracheobronchial tree; dysphagia due to esophageal compression; or the superior vena cava syndrome. Aneurysmal compression or erosion into adjacent bone may cause chest or back pain.
Aneurysmal compression of branch vessels or the occurrence of embolism to various peripheral arteries due to thrombus within the
aneurysm can cause coronary, cerebral, renal, mesenteric, lower extremity and rarely, spinal cord ischemia (SCI) and resultant symptoms.
aneurysm can cause coronary, cerebral, renal, mesenteric, lower extremity and rarely, spinal cord ischemia (SCI) and resultant symptoms.
The most serious complications of thoracic aortic aneurysm are dissection or rupture. A descending thoracic aortic aneurysm can rupture into the adjacent esophagus, producing an aortoesophageal fistula and presenting with hematemesis. Rupture is often catastrophic, being associated with severe pain and hypotension or shock.
DIAGNOSIS
Chest x-ray
Most (75%) of TAs are asymptomatic. As such their detection is usually an incidental finding on CXR or axial imaging study done for other reasons. On CXR the aneurysm produces a widening of the mediastinal silhouette, enlargement of the aortic knob, or displacement of the trachea from midline. Other reported features include displaced calcification, aortic kinking, and opacification of the aorticopulmonary window.
However, chest x-ray cannot distinguish an aneurysm from a tortuous aorta and many aneurysms are not apparent on the chest x-ray, so it is not the initial diagnostic modality of choice for TAs or TAAAs.
Echocardiography
Echocardiography is a useful tool for the diagnosis of thoracic aortic aneurysm according to ACC/AHA 2003 guidelines. Transthoracic echocardiography (TTE) is the preferred procedure, with transesophageal echocardiography (TEE) usually being performed only if the examination is incomplete or additional information is needed. There are, however, two settings in which TEE is preferred: for examination of the entire aorta, especially in emergency situations, and for imaging when coexistent dissection is suspected.
Among patients with known bicuspid aortic valves, the 2006 ACC/AHA guidelines on valvular heart disease recommend an initial TTE to assess the diameters of the aortic root and ascending aorta. Patients with aortic root or ascending aorta diameter greater than 4.0 cm should undergo serial evaluation of aortic root/ascending aortic size and morphology by echocardiography, computed tomography (CT), or magnetic resonance on a yearly basis. A lower threshold for ascending aorta diameter should be considered in patients of small stature.
CT and MRI
CT with intravenous contrast and magnetic resonance imaging (MRI) are the preferred tests to detect a thoracic aortic aneurysm, determine its size, and define aortic and branch vessel anatomy. MRI is preferred for aneurysms involving the aortic root.
CT scans with contrast have become the most widely used diagnostic tool. They rapidly and precisely evaluate the thoracic and abdominal aorta to determine the location and extent of the aneurysm and the relationship of the aneurysm to major branch vessels and surrounding structures. They can help accurately determine the size of the aneurysm and assess dissection, mural thrombus, intramural hematoma, free rupture, and contained rupture with hematoma.
Sagittal, coronary, and axial images may be obtained with three-dimensional reconstruction. Stent graft planning for endovascular descending TA repairs requires fine-cut images from the neck through the pelvis to the level of the femoral heads. The takeoff of the arch vessels is critical to determine the adequacy of the proximal landing zone, as is assessing the patency of the vertebral arteries, if the left subclavian artery should be covered by the stent graft. Assessment of the common femoral artery access is essential to determine the feasibility of large-bore sheath access. A spiral CT scan with 1-mm cuts and three-dimensional reconstruction with the ability to make centerline measurements is crucial to stent graft planning.
Among patients with known bicuspid aortic valves, the 2006 ACC/AHA guidelines recommend MRI or CT when morphology of the aortic root or ascending aorta cannot be accurately assessed by echocardiography. MRI or CT is reasonable in patients with bicuspid aortic valves and aortic root dilatation on echocardiography to further quantify severity of dilatation and involvement of the ascending aorta.
Contrast Angiography
Contrast angiography is the best method for evaluating branch vessel pathology. However, this procedure is invasive with potential nephrotoxicity from contrast medium and is unable to discern extraluminal aneurysmal size. It is being replaced with 3D CT scan reconstructed images.
NATURAL HISTORY
It is not well studied but risk of rupture has been reported between 32 and 68% for large aneurysms. Most of the patients with TAs have associated cardiovascular disease, which accounts for the most common cause of death along with rupture in these patients. One- and five-year survival rate reported 65 and 20%, respectively.
The size of the aneurysm is the most important determinant of rupture. Reported incidence of rupture is around 16% for 40 to 59 mm aneurysms and more than 30% for 60 mm and above. Very low risk in cases of size below 40 mm. Aneurysms may rupture at smaller sizes in patients with Marfan’s syndrome or other connective tissue disorders.
Reported rate of dissection or rupture ranged from 2 to 3 to 7%/y for aneurysms less than 50, 50 to 59, and >60 mm, respectively. Among patients with aneurysms >60 mm, the combined end point of rupture, dissection, or death occurred at a rate of 15.6%/y. The 5-year rate of survival was 56% in patients with aneurysms ≥60 mm.
Growth rates average 0.07 cm/y in the ascending aorta and 0.19 cm/y in the descending aorta. The rate of growth is related to the initial diameter with larger aneurysms growing more quickly. The rate of expansion was much greater for aneurysms more than 50 mm in diameter (7.9 vs. 1.7 mm/y in smaller aneurysms). The anatomic location of the aneurysm is another factor associated with the rate of expansion. Aneurysms located within the midportion of the descending aorta have the most rapid growth, while those in the ascending aorta have the slowest expansion rate, despite having the greatest initial diameter.
Elefteriades et al. published the natural history of thoracic aortic aneurysms and recommends elective repair of ascending aneurysms at 5.5 cm and descending aneurysms at 6.5 cm for patients without any familial disorders such as Marfan’s syndrome. These recommendations are based on the finding that the incidence of complications (rupture and dissection) exponentially increased when the size of the ascending aorta reached 6.0 cm (31% risk of complications) or when the size of the descending aorta reached 7.0 cm (43% risk). Patients with Marfan’s syndrome or familial aneurysms should undergo earlier repair, when the ascending aorta grows to 5.0 cm or the descending aorta grows to 6.0 cm.
MEDICAL MANAGEMENT OF ASYMPTOMATIC ANEURYSMS
Asymptomatic patients with an aneurysm are initially managed medically, while surgery is indicated for symptomatic patients and for asymptomatic patients with rapid aneurysm expansion or a diameter greater than 50 to 60 mm in diameter, depending on body size, cause of aortic dilation, and other clinical factors.
For smaller patients, including many women, we recommend elective repair for aneurysms greater than twice the size of the nonaneurysmal aorta (normal segment) or for those with rapid expansion, defined as growth of more than 0.5 cm during a 6-month interval.
In the asymptomatic patient, medical management includes Aggressive blood pressure control, including beta-blockers being part of the regimen, in an attempt to slow aneurysm growth.
Patient education about signs and symptoms of the TAs through patient education.
Serial imaging of the aneurysm to evaluate growth and structure. The preferred imaging technique is CT scanning. Imaging should be repeated at 6 months after the initial study then yearly after that if there is no significant change in size.
There has been a real proven benefit of beta-blockers on the rate of aneurysms expansion except in adults with Marfan’s syndrome. Betablockers are thought to act by decreasing left ventricular contractility (dp/dt) and shear stress. The goal systolic pressure is 105 to 120 mm Hg if tolerated. Beta-blockers are recommended for patients with bicuspid aortic valves and dilated aortic roots (diameter greater than 40 mm) who are not candidates for surgical correction and who do not have moderate or severe aortic regurgitation.
Beta-blocker therapy is recommended in patients with thoracic aortic aneurysm who are being followed nonoperatively and they are the preferred drug for the treatment of hypertension or angina.
Doxycycline effect has been investigated as a direct MMP inhibitor with some inhibitory effect on aneurysms expansion.
Smoking cessation, aspirin, and statins should be considered in all patients including preoperative workup.
SURGICAL THERAPY
The optimal timing of surgery for a thoracic aortic aneurysm is uncertain since the natural history is variable, particularly for aneurysms less than 50 mm in size, and the majority of patients have concomitant cardiovascular disease that increases the risks associated with surgery.
Indications
The indications for surgery include:
The presence of symptoms.
An end-diastolic aortic diameter of 50 to 60 mm for an ascending aortic aneurysm and 60 to 70 mm for a descending aortic aneurysm; often ≥70 mm in high-risk patients.
Aortic size index (aortic diameter [cm] divided by body surface area [m2]) for the ascending aorta, patients are stratified into three groups: ASI < 2.75 cm/m2 are at low risk for rupture (4%/y), ASI 2.75 to 4.25 cm/m2 are at moderate risk (8%/y), and ASI > 4.25cm/m2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree