Approach to and Management of Intermittent Claudication
Approach to and Management of Intermittent Claudication
Rekha Durairaj
Sanjay Rajagopalan
INTRODUCTION
Incidence and Prevalence of Peripheral Artery Disease
Lower extremity atherosclerotic peripheral artery disease (PAD) is commonly encountered in clinical practice. Although prevalence figures vary widely, assessment of ankle/brachial systolic pressure index (ABI) indicates that prevalence increases to 20% in individuals greater than 70 years. Of those with PAD, 10% have classic claudication, 50% have atypical leg pain, and the remaining 40% did not have exercise-induced leg pain. The relative risk of PAD in women versus men is 0.7. PAD seems to occur more frequently in Hispanics (relative risk, 1.5) and African Americans (relative risk, 2.5).
Risk Factors for Peripheral Arterial Disease and the Risk Conferred by Peripheral Artery Disease
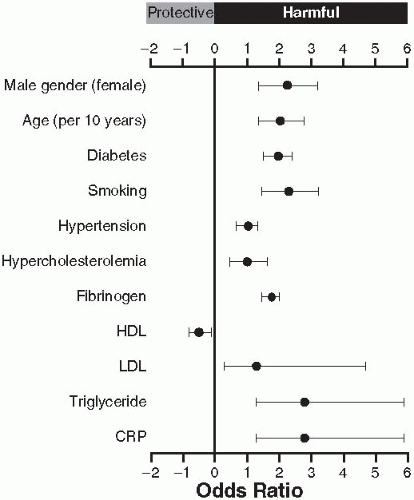
The risk factors for claudication are the same as those for atherosclerosis as outlined in
Figure 6.1. Smoking, male gender, age, diabetes, dyslipidemia in particular ratio of total to high-density lipoprotein (HDL) cholesterol (TC/HDL-C) and low HDL have strong associations with lower extremity atherosclerosis. Given the commonality of risk factors and the systemic nature of atherosclerosis, it is not surprising that patients with PAD have concurrent coronary artery and cerebrovascular disease.
Cardiovascular Risk with PAD
The 10-year risk of death in people diagnosed as having PAD is 40% and has remained largely unchanged since 1950. After multivariate adjustment for age, sex, and other risk factors for cardiovascular disease, patients with PAD had a threefold higher risk of all-cause death and a sixfold higher risk of cardiovascular-related death. Patients with an anklebrachial index (ABI) of less than 0.9 were found to have hazard ratios of 1.7 and 2.5 for all-cause and cardiovascular mortality, respectively. In patients with an ABI greater than 1.4 (indicative of poorly compressible vessels), the hazard ratios was 1.8 and 2.1 for all-cause and cardiovascular mortality, respectively. The REACH registry, an international consortium of practices examined the morbidity/mortality associated with vascular disease. The study involved 55,814 patients, the majority of whom were on evidence-based risk-reduction therapy. The 1-year incidence of cardiovascular death, MI, stroke and hospitalization was highest in patients with established PAD (21%) compared to 15% for CAD patients. The event rates increased with the number of symptomatic arterial disease locations, ranging from 5.3% for patients with risk factors only
to 13% for patients with one, 21% for patients with two, and 26% for patients with three arterial disease locations.
Progression to Limb Threat and Chronic Critical Limb Ischemia
The vast majority of patients with intermittent claudication (IC) (˜70%) do not alter their symptoms. Progression of symptoms occurs in 25% of cases over a 5-year period. The deterioration is most often during the first year after identification of cases (7 to 9%) compared with 2 to 3% per annum thereafter. Major amputation is relatively rare in IC, with 1 to 3% needing major amputation over a 5-year period. A changing ABI is possibly the best individual predictor of deterioration of PAD (e.g., need for arterial surgery or major amputation). In patients with IC in the lowest strata of ankle pressure (i.e., 40 to 60 mm Hg), the risk of progression to severe ischemia or actual limb loss is 8.5% per year.
Impact on Quality of Life
PAD does impact quality of life negatively, especially in patients with CLI. In a number of cases, the patients adapt subconsciously to a new workroutine to avoid provocation of symptoms.
CLINICAL FEATURES
Presenting Features
IC is classically defined as pain in one or both legs that occurs with walking or exertion, does not resolve with continued activity, and abates shortly (within 10 minutes) upon rest or a reduction in walking pace. Symptoms vary depending on the extent and levels of disease involvement but are commonly described as a cramping pain with or without muscle weakness. Recent observational studies demonstrate that greater than 50% of individuals with PAD are asymptomatic or report lower extremity symptoms that are atypical.
Pathophysiology
Regardless of the location of PAD within the lower extremity vasculature, claudication symptoms are most frequently localized to the muscles of the calf. This may be on account of the relatively greater metabolic demand and the common affliction of the superficial femoral artery (SFA) in atherosclerosis. The pathophysiology of IC is multifactorial and includes hemodynamic factors akin to coronary stenosis (e.g., a 70% stenosis may produce no symptoms at rest but may be flow-limiting with exercise). Other factors include deconditioning, metabolic changes including accumulation of acylcarnitines, impaired synthesis of phosphocreatine, and skeletal muscle injury characterized by a distal axonal denervation leading to muscle fiber loss and atrophy of affected muscles.
DIAGNOSIS
History and Physical Examination
Features that should be elicited include (a) the location of the pain or discomfort and duration of the symptoms, (b) the distance before (i) experiencing the discomfort (initial claudication distance) and (ii) being forced to stop (absolute claudication distance), (c) the elapsed time after exercise is stopped before the pain is relieved, and (d) the position of patient (standing at rest, sitting, lying) necessary to relieve the pain. Examination of the entire vascular system (looking for carotid and abdominal bruits) is mandatory with palpation of the radial, carotid, femoral, popliteal, dorsalis pedis, and posterior tibial artery pulses being crucial. In up to 10% of cases, pedal pulses may not be palpable. In such cases, the lateral tibial artery (branch of the peroneal) may be palpated below the ankle medial to the bony prominence of the fibula. Conversely, detection of arterial pulses does not preclude severe ischemia in cases of very distal occlusions, which may occur as part of the cholesterol embolization syndrome or in diabetic patients.
Differential Diagnosis
IC may mimic other conditions and vice versa.
Chapter 1 (
Table 1.1) lists the most common conditions that may present as hip, calf, or foot claudication. Although PAD is the most common etiology for IC, occasionally other conditions may present with IC (
Table 6.1). IC should be distinguished from pseudoclaudication secondary to lumbar spinal canal stenosis. Features that distinguish true claudication from pseudoclaudication include sharp and paresthetic nature of the discomfort, pain with variable walking distance and relief with sitting or leaning forward.
Laboratory Testing in the Claudicant
The following tests should be routinely performed in all patients with claudication: CBC with platelets, fasting blood glucose, HbA1c levels, renal function (creatinine and BUN), fasting lipid profile, urinalysis (for microalbuminuria), and a 12-lead EKG. Concomitant testing (if indicated) for carotid stenosis (duplex exam) and coronary disease (if indicated) may be performed. In the atypical patient (premature disease, personal, or family history of thrombosis), one should consider a comprehensive workup for premature vascular disease including hypercoagulability as outlined in
Table 6.2.
Diagnostic Modalities and Approach to Intermittent Claudication
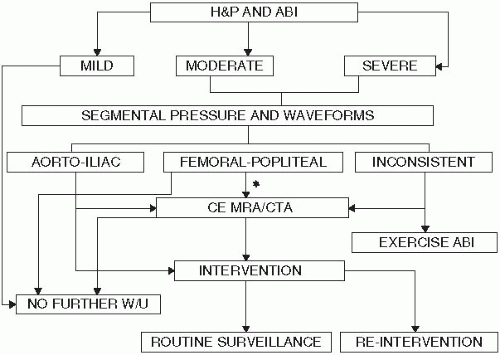
Figure 6.2 provides an initial diagnostic approach toward PAD. An ABI is often the first screening test in suspected PAD. Indices between 0.4 and 0.9 are associated with increasing disease severity. The ABI may be defined as the highest ankle pressure although recent studies suggest that the adoption of the lower of the two ankle pressures may provide for even better prognostic information. Segmental limb pressures, which may be obtained readily in the noninvasive vascular lab, may help localize the disease in the majority of patients with abnormal ABI. Exercise ABIs may be obtained in situations where the diagnosis is uncertain. Imaging with magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) may provide additional information in patients who are candidates for endovascular or surgical revascularization. In addition, these studies may be indicated where the diagnosis is uncertain. A false high normal ABI of greater than 1.3 can be associated with PAD in patients with diabetes mellitus when calcification of the media prevents compression of the artery by the cuff. In this situation, a toe brachial index of less than 0.7 will confirm the presence of PAD.
MANAGEMENT
The goals of treatment and management of patients with IC is to reduce cardiovascular risk, to relieve lower extremity symptoms, and to improve functional walking capacity and quality of life.
Risk Factor Modification
All patients with IC need intensive risk factor modification with careful attention paid to the factors outlined in
Table 6.3. Patients with PAD
cover the gamut of risk with some individuals carrying disproportionate risk compared to others. Appropriate identification of individuals at “high-risk” may be beneficial with the intention of more aggressive targets in such individuals. However, data demonstrating a survival benefit for PAD patients who have received aggressive risk modification are lacking. PAD at “very high risk” may be defined as the involvement of multiple
vascular beds (with a direct increase in risk based on the number of vascular beds involved); established PAD plus multiple ongoing major risk factors especially poorly controlled diabetes and smoking; PAD with prior intervention(s), especially surgical revascularization.
Smoking Cessation
Smoking cessation slows the progression of IC to critical limb ischemia, reduces need for revascularization, improves graft patency, and reduces cardiovascular events. All patients with IC should be referred to a smoking cessation program with use of nicotine replacement therapies (NRTs) and intensive counseling. NRT is dispensed as a gum (2 or 4 mg), nasal spray (0.5 mg/dose), patch (7, 14, and 21 mg), lozenge (2, 4 mg, typically), or inhaler (10 mg/cartridge with 4 mg/delivered dose). In general, various forms of NRT increase the likelihood of smoking cessation when used alone at least in the short-term. Evidence also indicates that the nicotine patch combined with another NRT is more effective than any single NRT. An advantage of NRT over other pharmacotherapy is their over-the-counter availability and flexible dosing. NRT can also be combined with other pharmacotherapy such as Bupropion. Bupropion is begun at 150 mg q.d. for 3 days, then increasing to b.i.d. for 6 to 10 weeks and longer if needed. Treatment with sustained-release bupropion in combination with NRT may not necessarily result in higher rates of cessation.
Varenicline is a non-nicotinic partial agonist of the α4β2 nicotinic receptors. Varenicline binding to these receptors leads to partial stimulation of receptor-mediated release of dopamine in the reward center in the brain and competitive inhibition of receptor binding by nicotine delivered from cigarettes. Data from three trials suggest that varenicline is more effective than sustainedrelease bupropion at least at 12 weeks of therapy. The recommended dosage schedule for varenicline is 0.5 mg q.d. for 3 days then twice daily for 4 days, increased to 1 mg twice daily for 7 to 12 weeks. Caution should be exerted with this drug in patients with neuropsychiatric conditions and seizures.
Correction of Hyperlipidemia
Treatment of hyperlipidemia prevents the progression of peripheral vascular atherosclerosis. Lipid lowering therapy may improve claudication distance at least on the basis of small trials. Statins are mandatory in patients with PAD by virtue of their effects in reducing CV events as shown initially in the Heart Protection Study that enrolled 6,748 patients (out of 20,500 patients) with PAD. The target LDL for PAD patients is less than 70 mg/dL. Additional medications (
Table 6.3) may be required alone (e.g., individuals intolerant of statins) or in combination therapy to achieve LDL-C goals. Insulin resistance typified by an elevated triglyceride level in the setting of a low HDL-C is common in PAD. A secondary lipid goal in the IC patient is to achieve an appropriate target non-HDL-C level (total cholesterol—HDL-C) of less than 130 mg/dL in such patients. Patients who require the addition of niacin or fibric acid therapy to statins (
Table 6.3) to achieve goals should undergo appropriate monitoring of liver function tests. Statins have been shown in small trials to improve claudication symptoms and objective parameters of treadmill walking. Niaspan (sustained release niacin) in conjunction with statin did not, however, appear to improve claudication distance in PAD over a duration of 6 months. The benefit of Niaspan in Type II DM patients and in metabolic syndrome (with Lapropripant) is being tested in AIM-HIGH and HPS-THRIVE studies, respectively.
Hypertension Control
The presence of PAD is evidence of cardiovascular “target organ disease” that should prompt acceleration of the pace of pharmacologic antihypertensive therapy with blood pressure goals of less than 140/90 mm Hg in most patients and perhaps a goal of less than 130/80 mm Hg in the diabetic (although ACCORD-BP questions this goal, the low event rates in these trials and the sample size complicates definitive interpretation). A goal of less than 130/80 may also be reasonable in the CKD patient. The effect of BP control on PAD progression or IC symptoms remains uncertain. The addition of an ACE inhibitor/ARB, especially in the PAD patient with concomitant diabetes, is reasonable. The Heart Outcomes Prevention Evaluation trial showed that ramipril protected against cardiovascular events beyond the extent expected from blood pressure lowering alone. There is also evidence that ACE inhibition may increase pain-free and maximum walking time in patients with symptomatic PAD. The typical PAD patient may require multiple medications and a combination of ACEI with calcium channel blockers may be particularly beneficial in both diabetics and nondiabetics. The use of beta-blocker medications may also be indicated in the PAD patient with concomitant coronary artery disease and/or angina. There is no contraindication to using beta-blockers in these patients.
Glycemic Control
Diabetics with IC have an overall amputation risk of 20% and a 5-year mortality of 50%. These patients have not been formally studied as a group in clinical trials and thus information is based on Type II DM
patients. Intensive blood glucose control (HbA1c < 7) while beneficial for microvascular complications, may not provide macrovascular benefits in Type II DM within 5-years. A tighter goal may be reasonable in specific patients who are not on hypoglycemia-inducing drugs, do not have evidence of advanced disease, and are involved in their own care. In most PAD patients, a goal of 7 may be reasonable. Meticulous attention to foot care is necessary to reduce the risk of skin ulceration, necrosis, and subsequent amputation. The role of newer therapies such as GLP-1 analogues and DPP-IV inhibitors needs to be studied.
Antiplatelet Therapy and Anticoagulation for Cardiovascular Risk Reduction
Aspirin should be used in dosages between 81 and 325 mg daily in patients with symptomatic PAD as part of overall cardiovascular prevention. On the basis of data from the Antiplatelet Trialists’ meta-analysis, aspirin may also reduce the need for peripheral revascularization and increase graft patency postsurgery. Compared to aspirin treatment, use of clopidogrel (an ADP receptor antagonist) results in a relative risk reduction of 24% for major adverse cardiovascular events compared to ASA alone in a prespecified subgroup of PAD in the CAPRIE trial. The data for antiplatelet therapy in the primary prevention of events in the asymptomatic patient population or in those with Type II diabetes or risk factors alone (absence of PAD) are more controversial.
Table 6.4 summarizes the data from trials since 2008 that have attempted to address this question. A key issue with a number of these trials, at least in Type II diabetes and PAD is their small sample size and that a lower dose of ASA was typically used. Ongoing clinical trials, such as A Study of Cardiovascular Events in Diabetes, which involves 10,000 patients with diabetes, and the Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D) may have adequate statistical power to detect a significant treatment effect of aspirin beyond contemporary background therapy.
There is no indication to use systemic anticoagulation therapy in patients with PAD without bypass grafts based on the WAVE trial that also showed increased fatal bleeding in this population (
Table 6.4).
Antithrombotic/anticoagulation in Peripheral Artery Disease Post Lower Extremity Bypass Grafting
Postoperatively, treatment with aspirin at 325 mg is recommended in all patients to decrease graft occlusion, as well as for its cardio protective effects. A study comparing infrainguinal autologous bypass with and without adjunctive ticlopidine has shown superior graft patency rates in those receiving this antiplatelet agent. In the more recent CASPAR, although there was no benefit of clopidogrel + ASA (75 to 100 mg) in below-knee bypass grafts in the main trial compared to ASA. In a prespecified subgroup of below-knee prosthetic grafts, there was a benefit of dual antiplatelet therapy in patients with prosthetic (HR, 0.65; 95% CI, 0.45-0.95;
P = 0.025), but not venous, grafts. It may be beneficial to anticoagulate patients with a vitamin K antagonist, such as warfarin, in high-risk grafts prone to thrombosis, such as those with grafts to below the knee locations, at least for the first 3 months following bypass with continued therapy mandated by the presence of other risk factors for continuing thrombosis. In the BOA trial, however, there was no benefit of oral anticoagulation in infrainguinal grafts with a particular lack of benefit in prosthetic grafts (also see section on anticoagulation in
Chapter 7).