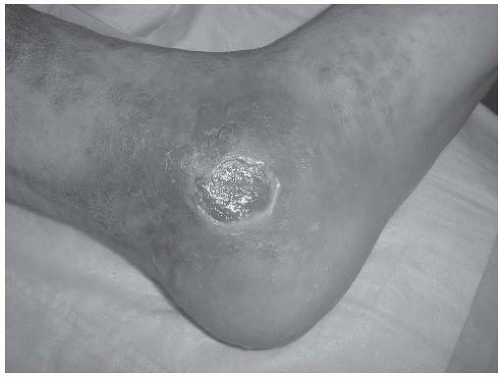
tortuous, allowing extravasation of fluid, red blood cells, and macromolecules. There is an increase in white blood cell adhesion to the endothelium as well as leukocyte activation. Increased microvascular blood flow is seen initially, but there can be late findings of capillary thrombosis leading to tissue hypoxia. Within the tissues, the extravasated red blood cells break down to produce a dark pigment called hemosiderin. The perivascular space becomes surrounded by intracellular matrix proteins. Chronic accompanying inflammation leads to scarring and fibrosis of the subcutaneous tissues. Macrophages, mast cells, and T lymphocytes are the cell types most commonly involved in tissue destruction. As skin perfusion decreases, the resulting ischemia can lead to skin breakdown and ulceration.
TABLE 22.1 RISK FACTORS FOR DVT | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
scoring system and Venous Clinical Severity Score (VCSS) system have been described in order to objectively describe the clinical response. The Villalta scale gives 0 (none) to 3 (severe) points for five patient-given symptoms and six physician-observed signs. A score greater than 5 is consistent with the postthrombotic syndrome and has been shown to correlate with quality of life. The VCSS also awards 0 (none) to 3 (severe) for ten different complaints (see Table 22.3). These systems are recommended by various organizations including the American Venous Forum in order to standardize reporting and allow more objective observation of clinical benefit and improved research.
TABLE 22.2 CEAP CLASSIFICATION | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
include the need for an experienced vascular technologist to perform the exam, interoperator variability, and the inability to accurately visualize the venous system above the inguinal ligament. Duplex ultrasound provides direct evaluation of the veins in the lower extremity and indirect evaluation of the pelvic vasculature. The evaluation of the iliac veins through ultrasound relies on indirect evidence of obstruction such as loss of characteristic cessation of venous flow with Valsalva maneuver and loss of respiratory variation, indicating a more proximal obstruction.
TABLE 22.3 VENOUS CLINICAL SEVERITY SCORE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
tilted 60 degrees, while contrast is injected through a catheter with the tip placed at the distal external iliac vein. Gravity and a Valsalva maneuver propel the contrast distally in limbs with incompetent valves. This allows grading of Phlebographie reflux as well as delineating competent valve cusps, which are clearly outlined with this technique. Reflux grades include 0, no reflux; 1, reflux into the femoral vein to the level of the proximal thigh; 2, reflux into the femoral vein but not through the popliteal vein; 3, reflux to a level just below the knee; and 4, reflux through to the level of the calf or ankle. However, descending phlebography requires an experienced imaging team to perform and is costly. Duplex scanning obviates the need for this test in many circumstances, although phlebograms are often performed in conjunction with therapeutic maneuvers such as venoplasty and stenting as well as thrombolysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree