, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
Systemic artery-to-pulmonary artery shunts are palliative surgical procedures performed to increase the blood flow to the lungs for the relief of cyanosis and to enlarge the pulmonary arteries. Palliative procedures improve oxygen saturation, but they also can result in volume loading of the systemic ventricle and possible pulmonary hypertension.
22.1 Indications
Systemic artery-to-pulmonary artery shunts are palliative surgical procedures performed to increase the blood flow to the lungs for the relief of cyanosis and to enlarge the pulmonary arteries. Palliative procedures improve oxygen saturation, but they also can result in volume loading of the systemic ventricle and possible pulmonary hypertension.
22.2 Blalock–Taussig Shunts
The original (classic) Blalock–Taussig (BT) shunt (described in 1945) involved ligation and division of the left subclavian artery and an end-to-side anastomosis of the proximal left subclavian artery to the pulmonary artery. Restitution of flow in the ipsilateral upper limb was dependent on collateral vessel formation. Disadvantages of the classic BT shunt included possible distortion or kinking of the subclavian or pulmonary arteries at the anastomotic sites and the potential for abnormal growth of the arm ipsilateral to the shunt (due to a steal phenomenon). The classic BT shunt is now rarely used for palliative surgery.
The subsequent, modified Blalock–Taussig (MBT) shunt uses a synthetic graft of polytetrafluoroethylene to create a side-to-side shunt between the subclavian artery and ipsilateral right or left pulmonary artery (Figs. 22.1 and 22.2). Advantages of the MBT include less distortion of the pulmonary arteries (although it can still occur), better growth of the pulmonary tree, and preserved blood flow to the ipsilateral arm. Distortion of the ipsilateral pulmonary artery has been reported in 24–33 % of patients, hemodynamically significant pulmonary artery stenosis in 14 % of patients, and shunt narrowing at the anastomosis in 50 % of patients [1, 2]. Rarely, there is complete occlusion of the pulmonary artery or the shunt [1, 2]. Another not uncommon complication of the MBT shunt is shunt thrombosis (Fig. 22.3). This is usually a late complication but can occur in the early postoperative period.
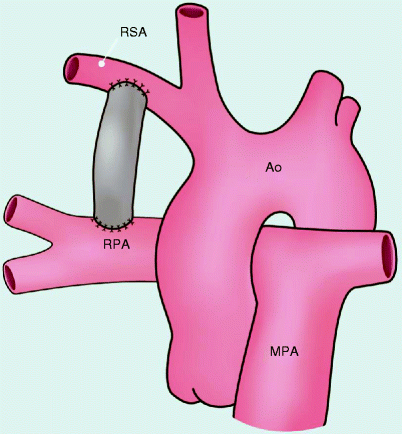
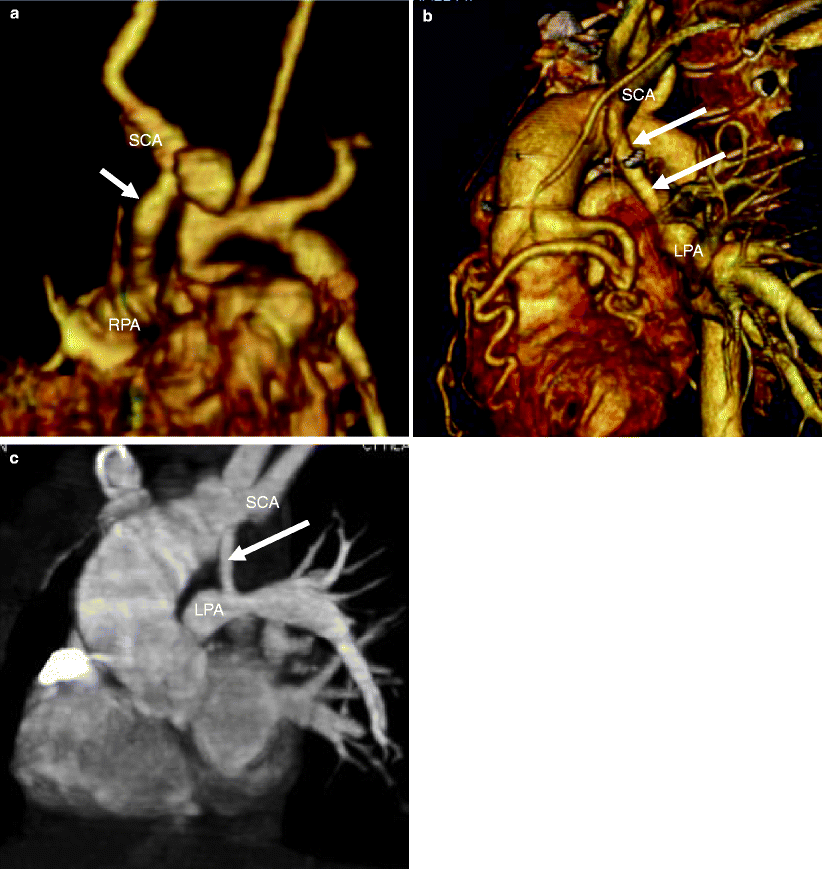
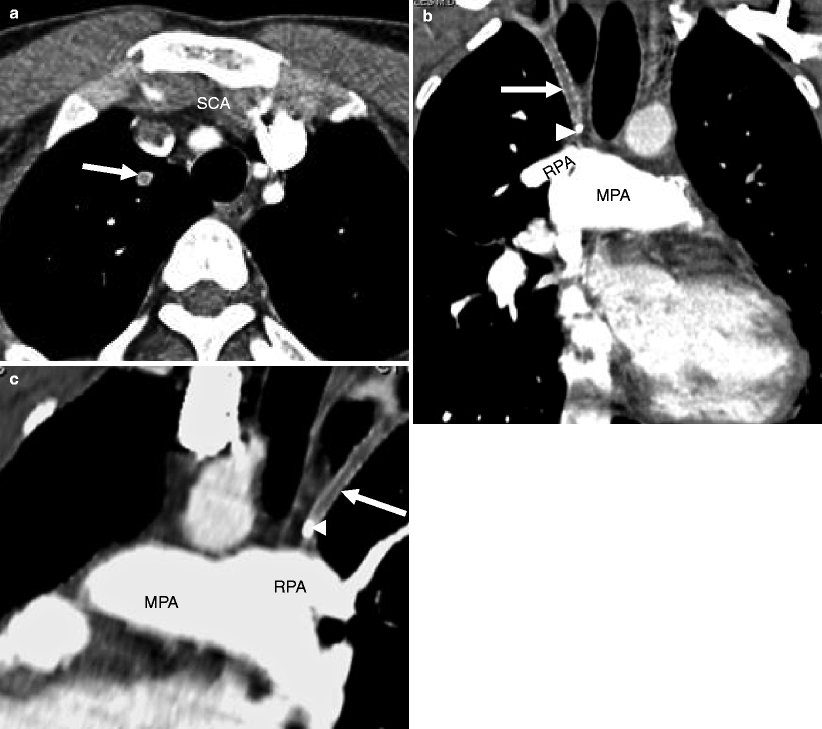

Fig. 22.1
Modified Blalock–Taussig shunt. This procedure involves interposition of a prosthetic graft of polytetrafluoroethylene between the subclavian artery and ipsilateral right or left pulmonary artery. Ao aorta, MPA main pulmonary artery, RPA right pulmonary artery, RSA right subclavian artery

Fig. 22.2
Modified Blalock–Taussig shunt. Panel (a) is a 3D reconstruction illustrating the shunt between the right subclavian artery and right pulmonary artery. Panels (b) and (c) are 3D views in two separate patients showing a shunt between the left subclavian artery and the left pulmonary artery. Arrow Blalock–Taussig shunt, RPA right pulmonary artery, LPA left pulmonary artery, SCA right subclavian artery

Fig. 22.3




Shunt thrombosis in a patient with single-ventricle physiology. Panel (a) is an axial view showing no flow in the Blalock–Taussig shunt (arrow). Panel (b) is a coronal cut and panel (c) is an oblique sagittal view. In panels (b) and (c), the Blalock–Taussig shunt (arrow) extends from the right subclavian artery to the right pulmonary artery (RPA) with a metallic clip (arrowhead) noted near its entrance to the right pulmonary artery. Flow is absent in the shunt consistent with thrombosis. MPA main pulmonary artery, SCA subclavian artery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


