Baseline
Long term survival
Follow up
Author
Year
Ref
Subgroups
N
LVEF
Mean gradient
AVA
NYHA class III–IV
1 year
3 year
5 year
10 year
NYHA class III–IV
(%)
(mmHg)
(cm2)
(%)
(%)
(%)
(%)
(%)
(%)
Monin
2003
[4]
Contractile reserve
28
31(23–35)
27(22–35)
0.7(0.6–0.9)
85
70a
n/a
20a
82
No Contractile reserve
13
30(27–35)
30(23–34)
0.8(0.7–0.9)
83
35a
n/a
12a
93
Varadarajan
2006
[5]
All
453
52 ± 21
40 ± 16
0.71 ± 0.17
n/a
62
32
18
CHF
189
50
20
Systolic PAP ≥ 60
83
38
<20
LVEF ≤40 %
159
40
20
3–4+ mitral regurgitation
118
50
25
Brown
2008
[6]
90
60 ± 12
n/a
0.9 ± 0.3
n/a
50
15
2
Clavel
2008
[7]
57
29 ± 8
21 ± 8
0.92 ± 0.2
47
70a
50a
Pai
2008
[8]
Low LVEF (<35 %)
136
24 ± 0.8
33 ± 14
0.67 ± 0.18
n/a
47
n/a
23
n/a
n/a
Low gradient (<30 mmHg)
121
39 ± 19
24 ± 6
0.76 ± 0.15
n/a
60
40(2 years)
22
n/a
Tribouilloy
2009
[9]
26
27 ± 6
24 ± 6
0.74 ± 0.18
81
35a
13 ± 7
n/a
n/a
Kapadia
2015
[10]
179
51 ± 14
43 ± 15
0.6 ± 0.2
93
49
11
6.4
n/a
40
Aortic valve surgery is a well-established and reproducible procedure that is associated with low peri-procedure morbidity and mortality, symptomatic improvement, and improvement in long-term survival [1, 12]. In spite of its safety and benefits, a large proportion of patients with CHF secondary to aortic valve disorders don’t have surgery. Reasons for no intervention include too advanced cardiac disease, advanced age, presence of comorbidities, and short life expectancy [2, 13]. The notion that surgery is associated with prohibitively high operative risk and no significant clinical improvement in patients with advanced heart failure secondary to aortic valve disease dissuade many practitioners to recommend aortic valve replacement (AVR). In this chapter we review the indications for surgical management and the outcomes of patients with advance heart failure symptoms (NYHA class III–IV) and left ventricular dysfunction (LVEF ≤35 %) secondary to aortic valve stenosis and regurgitation. Notwithstanding their high operative risk, most of these patients benefit form AVR. AVR improve their symptoms, cardiac function, and long-term survival compared to medical management.
Aortic Stenosis and Congestive Heart Failure
Aortic stenosis is a disease of the elderly [14, 15]. It is estimated that 2.8 % of the population older than 70 years have aortic stenosis [14, 15]. Of them, 40–60 % have class III–IV symptoms and only one third of patients with LVEF ≤35 % have AVR [2, 8, 16].
Aortic stenosis leads to left ventricular outflow obstruction and chronic pressure overload of the left ventricle. The LV hypertrophies in order to decrease wall stress. The magnitude and adequacy of that hypertrophy and the associated changes in systolic ventricular function determine the clinical presentation, hemodynamic characteristics, response to treatment, and prognosis [17–20] (Fig. 10.1). Aortic stenosis can lead to heart failure symptom by several mechanisms: (1) Diastolic dysfunction: it is the result of LV hypertrophy, increased wall thickness and decreased LV volume to mass ratio. LV end diastolic pressure (LVEDP) is increases from diminished compliance and not from systolic failure [21–24]. (2) Systolic dysfunction secondary to afterload mismatch: if the hypertrophic process is inadequate to compensate for the increased afterload, wall stress increases and the ejection fraction falls. This condition is called “afterload mismatch” and limits fiber shortening [18–21]. There are two subgroups in this category: (a) patients that preserve their stroke volume and therefore their transaortic gradients are elevated and (b) patients on whom the stroke volume diminishes and therefore the transaortic gradient is low. This last group is difficult to differentiate from the next one. (3) Systolic dysfunction secondary to intrinsic myocardial dysfunction: Persistently elevated wall stress, inadequate blood supply, and superimposed ischemia or infarction, myocardial fibrosis, and abnormalities of calcium handling further depress myocardial contractility. As before, these patients have diminished stroke volume and low transvalvular gradients but the benefits of surgery are less well established [20, 21]. If myocardial dysfunction is secondary to afterload mismatch, AVR is associated with good outcomes. If intrinsic myocardial dysfunction predominates, the response to AVR is less favorable with higher operative mortality and less LVEF improvement after AVR [18, 20, 25, 26]. Nevertheless their less favorable outcome with AVR, these patients have a significantly better prognosis with surgery that with medical management.
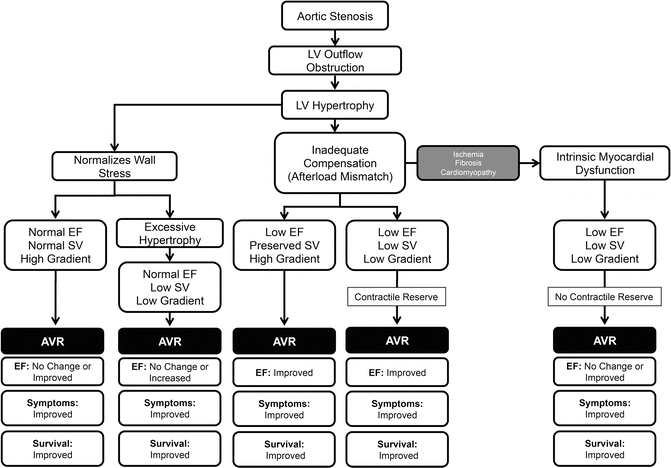

Fig. 10.1
Mechanisms responsible for heart failure in aortic stenosis and response to aortic valve replacement
CHF Secondary to Aortic Stenosis with Normal Left Ventricular Function and Normal Stroke Volume
If the LV hypertrophy is adequate, the wall stress normalizes and the left ventricular function is maintained (Fig. 10.1) [18, 19, 21]. These patients have normal left ventricular function as evidenced by a normal stroke volume and ejection fraction. The transvalvular gradient is elevated. LVEDP is elevated secondary to decreased compliance from diastolic dysfunction and increased afterload.
They respond very well to aortic valve replacement. The surgical risk is low [1, 12]. Risk adjusted operative mortality is 2.3 % and has steadily declined over the last 10 years [1]. The operative mortality increases with the severity of the symptoms and lower LVEF [1, 12]. Patients with congestive heart failure symptoms have an operative mortality of 4.4 % vs. 1.6 % on those without [1]. Operative mortality in patients with a LVEF ≥30 % is 2.4 % vs. 5.2 % if LVEF <30 % [1].
AVR effectively relieves symptoms and improves quality of life [27]. Long-term survival is similar to that expected for an age and sex matched population for patients with normal LVEF, but there is an excess mortality for patients with NYHA class III–IV symptoms [27–29]. Contemporary series have demonstrated that AVR can be performed with no operative mortality and 1 and 3-year survival of 97 and 94 % respectively [30]. Mihaljevic demonstrated in 3,049 patients operated for aortic stenosis that 5-year survival for patients with no LV dysfunction was 80 %. However, for those in NYHA class III–IV, 5-year survival was 65–70 %[28]. The New York State database demonstrated that 30-month survival for patients with EF >40 % was 87.5 % and with CHF was 83.4 % [31].
AVR decreases ventricular afterload and is associated with improved LVEF, regression of LV hypertrophy, and LV mass [25, 32, 33]. Sharma described that LVEF improved by 6.8 EF points after AVR. The improvement was evident at 6 months and was maintained for up to 10 years after surgery (EF 56 ± 4 % preoperatively, 63 ± 3 % at 0–6 months, 63 ± 5 % at 7–24 months, and 63 ± 4 at 25–120 months) [34]. Some studies showed no change in LVEF after AVR in patients with normal LVEF. LV mass regression was more marked in the first 6 months after surgery and maintained for up to 10 years (181 ± 26 g/m2 preoperative vs. 124 ± 27 g/m2 at 6 months, 117 ± 15 g/m2 at 24 months, and 113 ± 14 g/m2 at 120 months after AVR) [34].
CHF Secondary to Aortic Stenosis with Normal Left Ventricular Function and Low Transvalvular Gradient (AVA ≤0.8 cm2, EF ≥50 %, Mean Aortic Valve Gradient <40 mmHg) (Table 10.2)
Table 10.2
Severe aortic stenosis with normal left ventricular function and low transvalvular gradient: AVR vs. medical management
Preoperative | Surgery | Long term survival | Postoperative | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Author | Year | Ref | Subgroups | N | LVEF | Mean gradient | AVA | NYHA class III–IV | CABG | 30 day mortality | 1 year | 3 year | 5 year | 10 year | NYHA class III–IV | LVEF |
(%) | (mmHg) | (cm2) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |||||
Aortic valve replacement | ||||||||||||||||
Hachicha | 2007 | [38] | 80 | 62 ± 8 | 32 ± 17 | 0.76 ± 0.23 | n/a | n/a | n/a | >95a | 93 ± 3 | >80a | n/a | n/a | ||
Pai | 2008 | [8] | 18 | 66 ± 7 | 26 ± 5 | 0.77 ± 0.14 | n/a | 66 | n/a | 92 | 88 (2 years) | 88 | n/a | n/a | n/a | |
Tarantini | 2011 | [36] | 72 | 61(56–67) | 33(27–39) | 0.9(0.8–0.99) | 35 | 52 | 2.7 | 90a | 78a | 15 | 18 | n/a | ||
Herrmann | 2011 | [37] | 11 | 61 ± 5 | 33 ± 7 | 0.8 ± 0.2 | 100 | n/a | 18 | n/a | n/a | n/a | 100 | Improved by 3 % | ||
Medical management | ||||||||||||||||
Hachicha | 2007 | [38] | 91 | 62 ± 8 | 32 ± 17 | 0.76 ± 0.23 | – | – | 81a | 58 ± 8 | <40a | |||||
Pai | 2008 | [8] | 14 | 66 ± 7 | 26 ± 5 | 0.77 ± 0.14 | – | – | 82 | 10 | ||||||
Tarantini | 2011 | [36] | 29 | 60 (50–66) | 33 (27–37) | 0.90 (0.75–1.00) | 90 | – | – | 80a | 35a | |||||
These patients have more hypertrophy than the necessary to compensate for the increased afterload and wall stress (Fig. 10.1) [21]. This group represents 9–35 % of patients with severe AS and normal LVEF [8, 35–38]. They are commonly overlooked in clinical practice. Since they have preserved LVEF and low transvalvular gradient, the small AVA is often attributed to calculation error [35]. The severity of their stenosis is erroneously underestimated [35]. Therefore, they are 40–50 % less likely to be referred to surgery [35, 38].
These patients are often elderly females, have severe left ventricular hypertrophy, thicker ventricles, smaller left ventricular cavities with a restrictive filling pattern (diastolic dysfunction) and intrinsic myocardial dysfunction secondary to myocardial fibrosis [8, 35, 37, 39, 40]. The low transvalvular gradient results from decreased flow across the aortic valve secondary to low stroke volume or prolonged systolic ejection period [36]. These patients are in more advanced stages of their disease and have worse prognosis than patients with normal EF and high gradient aortic stenosis [38, 39].
Several studies have demonstrated that these patients have better survival when treated with AVR compared to medical management (Table 10.2). The operative mortality is between 2.7 % and 18 %. These patients are predisposed to low cardiac output postoperatively given their severe left ventricular hypertrophy and diastolic dysfunction, and decreased systemic arterial compliance [39]. Aggressive volume resuscitation and beta blockade is often necessary.
Pai studied 52 patients with severe aortic stenosis, EF ≥55 % and a mean transvalvular gradient <30 mmHg [8]. By propensity score matching 18 patients who had AVR were compared with 14 patients without AVR. One and 5-year survival were 92 % and 88 % in the AVR group compared with 82 % and 10 % in the non-AVR group. Series from Tarantini and Hachicha also confirmed those findings (Table 10.2) [36, 38]. LVEF an NYHA functional class improved after surgery [36].
CHF Secondary to Aortic Stenosis with Low Left Ventricular Ejection Fraction
Poor preoperative left ventricular function is the major predictor of outcomes in patients with aortic stenosis [25, 28, 29, 31].
The incidence of left ventricular dysfunction in patients with severe aortic stenosis is difficult to precise. It varies with the definition used and the population investigated. 5.4 % of patients in the Society of Thoracic Surgeons database who had isolated AVR between 1997 and 2006 had LVEF <30 % [1]. The Euro Heart Survey of Valvular Heart Disease showed that 2.9 % of the patients who underwent AVR had LVEF <30 % and 16.4 % had LVEF between 30 % and 50 % [2]. In AVR series, the incidence ranges from 12 % to 21 % depending on the LVEF threshold used [18, 41]. In a study from an echocardiography database, 26 % of patients with severe aortic stenosis had LVEF ≤35 % and 23 % had a mean transvalvular gradient ≤30 mmHg [8]. Only one third of them had AVR [8].
CHF Secondary to Aortic Stenosis with Low Left Ventricular Ejection Fraction and High Transvalvular Gradients (Table 10.3)
Table 10.3
AVR for severe aortic stenosis with left ventricular dysfunction and high transvalvular gradient
Preoperative | Surgery | Long term survival | Postoperative | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Author | Year | Ref | Subgroups | N | LVEF | Mean gradient | AVA | NYHA class III–IV | CABG | 30 day mortality | 1 year | 3 year | 5 year | 10 year | NYHA class III–IV | LVEF |
(%) | (mmHg) | (cm2) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |||||
Connolly | 1997 | [25] | 154 | 27 ± 6 | 44 ± 18 | 0.6 ± 0.2 | 88 | 51 | 9 | 82a | 60a | 58 | 7 | 39 ± 14 | ||
Powell | 2000 | [43] | 55 | 22 ± 6 | 41 ± 14 | 0.5 ± 0.2 | 84 | 55 | 18 | 77 % and 33 % without and with preop MI | Increased by 22 points in 95 % | n/a | ||||
Vaquette | 2005 | [44] | 155 | 25 ± 5 | 43 ± 13 | 0.6 ± 0.15 | 89 | 13 | 12 | >90a | 71 | 3 | 47 | |||
Matsumura | 2008 | [46] | 90 | 37 ± 10 | 42 ± 17 | 0.7 ± 0.2 | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a | 57 ± 11 | |
Pai | 2008 | [8] | Low LVEF (<35 %) | 58 | 26 ± 7 | 40 ± 16 | 0.64 ± 0.17 | n/a | 59 | 9 | 80 | n/a | 58 | n/a | n/a | n/a |
Flores Marin | 2009 | [42] | 82 | 33 ± 6 | 42 ± 18 | 0.58 ± 0.2 | 84 | 29 | 19.5 | 80a | 70 | 5 | n/a | |||
Halkos | 2009 | [45] | LVEF<40 | 119 | <40 | n/a | n/a | 42 | 45 | 10.9 | 82a | 62 | n/a | n/a | ||
LVEF 25–40 | 83 | n/a | n/a | n/a | 14.5 | |||||||||||
LVEF<25 | 36 | n/a | n/a | n/a | 2.7 | |||||||||||
These patients with CHF secondary to severe AS and depressed LVEF but able to generate transaortic gradients ≥40 mmHg, benefit significantly from AVR [8, 25, 41–45] (Fig. 10.1, Table 10.3). They represent 20 % of the patients with severe AS and low LVEF [41].
Thirty-day mortality ranged from 9 % to 19.5 %. Predictors of operative mortality were preoperative myocardial infarction, coronary artery disease, and cardiomegaly.
Symptomatic improvement occurred in the majority of patients after AVR. Most patients were in functional class I or II at late follow-up. LVEF improved early after AVR and continued to improve at late follow-up [34, 44]. The improvement in LVEF was usually more pronounced than in patients with preserved LVEF and severe AS [34]. Improvement in LVEF was associated with greater AS severity as determined by smaller aortic valve area and higher mean gradients, better preoperative ejection fraction, less remodeled ventricles, and the absence of coronary artery disease or previous myocardial infarction [25, 42, 46].
Aggregated long-term survival ranged from 77 % to >90 % at 1 year and from 58 % to 71 % at 5 years. In the absence of coronary artery disease survival of patients with severe aortic and reduced left ventricular function with elevated gradients was similar to the expected survival of the overall population [25]. Independent predictors of long-term survival by multivariate analysis are listed in Table 10.4.
Table 10.4
Risk factors associated with early mortality, long-term survival and improvement in LVEF after aortic valve replacement for low left ventricular ejection fraction low gradient aortic stenosis
Independent risk factors associated with 30 day mortality after AVR for low LVEF-low gradient aortic stenosis | |||||
Author | Ref | Factor | HR or RR | 95 CI | Association |
Coronary artery disease | |||||
Powell | [43] | Previous myocardial infarction | 14.9 | 2.4–92.1 | Positive |
Levy | [47] | Multivessel coronary artery disease | 2.2 | 1.02–5.02 | Positive |
Connolly | [25] | 4.6 | 1.4–15 | Positive | |
Flores Marin | [42] | 2.09 | 1.261–51 | Positive | |
Tribouilloy | [9] | Concomitant CABG | 9.7 | 1.9–49.9 | Positive |
Rothenburger | [48] | 4.12 | 0.94–18.7 | Positive | |
Myocardial dysfunction | |||||
Tribouilloy | [9] | Mean aortic valve gradient ≤20 mmHg | 10 | 1.2–84.9 | Positive |
Monin | [4] | 4.7 | 1.1–21 | Positive | |
Levy | [47] | Preoperative mean aortic valve gradient | 0.89 | 0.83–0.96 | Positive |
Levy | [47] | Absence of contractile reserve | 4.4 | 1.1–17.5 | Positive |
Monin | 10.9 | 2.6–43.4 | Positive | ||
Rothenburger | [48] | LVESD>54 mm | 0.24 | 0.05–1.05 | Positive |
Vaquette | [44] | Cardiothoracic ratio ≥0.6 | 12.2 | 5.4–27.4 | Positive |
Flores Marin | [42] | Preoperative mitral regurgitation | 2.37 | 1.44–80 | Positive |
Rothenburger | [48] | NYHA class III or IV | 0.14 | 0.02–1.12 | Positive |
Comorbidities and other factors | |||||
Halkos | [45] | Age | 1.05 | 1.01–1.08 | Positive |
Flores Marin | [42] | Female gender | 2.6 | 2.2–89 | Positive |
Rothenburger | [48] | Creatinine ≥1.4 | 11 | 2.34–56.82 | Positive |
Halkos | [45] | Emergent status | 5.9 | 1.21–28.08 | Positive |
Halkos | [45] | Cardiopulmonary bypass time | 1.03 | 1.01–1.03 | Positive |
Connolly | [26] | Small prosthesis | n/a | ||
Independent risk factors associated with long term survival after AVR for low LVEF–low gradient aortic stenosis | |||||
Author | Ref | Factor | HR or RR | 95 CI | Association |
Aortic valve replacement | |||||
Monin | [4] | AVR | 0.3 | 0.17–0.53 | Positive |
Pai | [8] | 0.5 | 0.3–0.87 | Positive | |
Tribouilloy | [9] | 0.16 | 0.12–3.16 | Positive | |
Pereira | [54] | 0.19 | 0.09–0.39 | Positive | |
Coronary artery disease | |||||
Levy | [47] | Multivessel coronary artery disease | 1.85 | 1.05–2.72 | Negative |
Tribouilloy | [9] | 1.3 | 1.08–2.07 | Negative | |
Connolly | [25] | n/a | Negative | ||
Pai | [8] | Concomitant CABG | n/a | Negative | |
Myocardial dysfunction | |||||
Monin | Contractile reserve | 0.4 | 0.23–0.69 | Positive | |
Levy | [47] | Preoperative mean aortic valve gradient >20 mmHg | 0.95 | 0.91–0.99 | Positive |
Tribouilloy | [9] | Preoperative mean aortic valve gradient ≤20 mmHg | 11.25 | 1.83–14.7 | Negative |
Connolly 97 | [25] | Preoperative low cardiac output | n/a | Negative | |
Flores Marin | [42] | Postoperative low cardiac output | 4.4 | 1.20–15.5 | Negative |
Tarantini | [83] | LVESVI ≤ 90 ml/m2 | n/a | Positive | |
Vaquette
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||