Aortic Valve Disease
Olcay Aksoy
Brian P. Griffin
I. AORTIC STENOSIS
A. Introduction.
Aortic stenosis (AS) causes progressive obstruction of the left ventricular outflow tract (LVOT), resulting in pressure hypertrophy of the left ventricle and the classic symptoms of heart failure, syncope, and angina pectoris. Stenosis most commonly occurs at the level of the valve (AS); however, subaortic and supravalvular stenoses are also well-defined entities. Untreated AS is associated with significant morbidity and mortality. As prompt recognition and treatment are associated with normalized life expectancy in those patients with symptomatic severe AS, careful evaluation and management can have significant impact on survival.
B. Etiology
1. Valvular AS has several causes, including congenital, rheumatic, bicuspid, and most commonly inflammation with resultant calcification.
a. The most common cause of AS in the United States is calcific degeneration. Although initially thought to be the result of normal “wear and tear” of the valve leaflets, there is now ample evidence that suggests that the progression of stenosis is related to an active process of inflammation involving the reninangiotensin system, lipid accumulation, and resultant calcification. Several inflammatory pathways are implicated, including those that utilize osteopontin, bone morphogetic proteins, and receptor activator of nuclear factor-κB ligand.
Aortic sclerosis is caused by calcification and thickening of the aortic valve without the increased gradients as seen in AS. Both aortic sclerosis and calcific aortic stenosis have been associated with traditional risk factors for atherosclerosis, such as smoking, hypertension, and hyperlipidemia. Aortic sclerosis is associated with an increased risk of cardiovascular death and myocardial infarction and can progress to AS. Other conditions associated with calcific AS include Paget’s disease and end-stage renal disease.
b. Bicuspid valves are present in 1% to 2% of the population, are predominant in men, and occur in 9% of first-degree family members of those afflicted. The most common anatomic abnormality seen in a bicuspid valve is the fusion of the right and left coronary cusps. Concurrent coarctation, aortic root dilation, and a propensity for aortic dissection are seen in a minority of patients. Severe stenosis typically develops by the fifth or sixth decade; however, earlier and later presentations are not uncommon. Unicuspid valves open only at one commissure, are uncommon, and are usually severely stenotic at an early age.
The most recent American College of Cardiology/American Heart Association (ACC/AHA) valve guidelines (2006) highlight the importance of evaluating the aorta and valve in bicuspid aortic valve with echocardiography or, if this is inadequate, with other imaging modalities such as magnetic resonance imaging or computed tomography. In some instances, the aorta may require surgical intervention before the valve. The guidelines recommend that patients with an aortic diameter > 4 cm and bicuspid valve should have
yearly follow-up of aorta size and should have surgical intervention if the aorta is > 5 cm or increases by > 0.5 cm in a year, irrespective of the valve lesion severity. If the valve requires surgery, then aorta replacement is recommended if the aorta is > 4.5 cm at the time of surgery. β-Blockers should be considered in bicuspid aortic valve patients in the presence of ascending aorta enlargement.
yearly follow-up of aorta size and should have surgical intervention if the aorta is > 5 cm or increases by > 0.5 cm in a year, irrespective of the valve lesion severity. If the valve requires surgery, then aorta replacement is recommended if the aorta is > 4.5 cm at the time of surgery. β-Blockers should be considered in bicuspid aortic valve patients in the presence of ascending aorta enlargement.
c. Rheumatic AS often coexists with aortic regurgitation (AR) and mitral valve lesions. It is a rare cause of isolated severe AS in the industrialized world. Fusion of the commissures occurs leaving a small central orifice.
2. Subvalvular AS. This is a congenital condition, although it may not be apparent at birth. Typically, a circumferential fibromuscular membrane involving the anterior mitral valve leaflet is present in the LVOT below the aortic valve. In more extreme cases, a tunnel-like obstruction may be present, rather than a discrete membrane. The pathogenesis of this condition is not perfectly understood but is thought to represent a maladaptive response to abnormal flow dynamics in the LVOT. It may exist with other left-sided obstructive lesions, such as coarctation as part of Shone’s syndrome. The condition may recur even after successful membrane resection. Subvalvular AS may be difficult to distinguish from hypertrophic cardiomyopathy, especially when secondary left ventricular hypertrophy (LVH) is pronounced.
3. Supravalvular AS is uncommon. It may occur as part of a congenital syndrome such as Williams syndrome caused by a mutation in the elastin gene (i.e., associated hypercalcemia, elfin facies, developmental delay, small stature, and multiple stenoses in the aorta and peripheral arteries) or be caused by lipid deposits in severe forms of familial hypercholesterolemia. Obstruction occurs above the valve in the ascending aorta.
C. Pathophysiology
1. Pressure overload. All forms of AS are characterized by progressive narrowing of the LVOT. To maintain cardiac output in the face of increased afterload, the left ventricle must generate higher systolic pressures, which increases LV wall stress. In response to the pressure overload and increased wall stress, the left ventricle undergoes compensatory, concentric hypertrophy. The increase in LV wall thickness allows the wall stress to normalize according to Laplace’s law: wall stress = (pressure × radius)/(2 × thickness).
2. Diastolic dysfunction. LV diastolic function is determined by LV relaxation properties and LV compliance (i.e., change in volume with change in pressure [dV/dP]). Increased afterload and LVH lead to a reduction in LV compliance. Furthermore, there are changes in strain and torsion characteristics of the left ventricle in AS. Passive early diastolic filling is reduced, and maintenance of an adequate LV preload becomes more dependent on active left atrial contraction.
3. Supply—demand mismatch. Myocardial oxygen demand is determined by heart rate, cardiac contractility, and myocardial wall stress. Over time, LVH is unable to compensate for the increase in wall stress imposed on the left ventricle by progressive pressure overload. As the AS becomes more severe, wall stress and myocardial oxygen demand increase in parallel. Concurrently, AS is associated with a decrease in myocardial oxygen supply. Progressive LVH and diastolic dysfunction lead to an elevation in left ventricular end-diastolic pressure (LVEDP). Elevated LVEDP leads to decreased perfusion pressure across the coronary bed and causes endocardial compression of small intramyocardial arteries, impairing coronary flow reserve. The imbalance between myocardial oxygen supply and demand can precipitate ischemia during exertion.
D. Natural history.
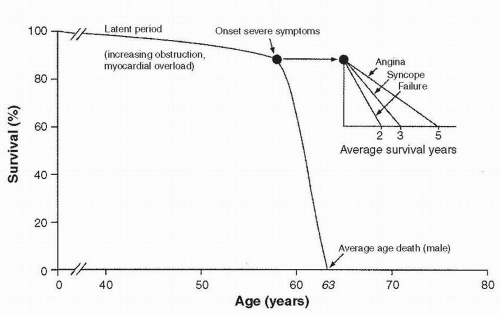
The classic survival curve for patients with untreated AS, as described by Ross and Braunwald (1), is shown in Figure 15.1.
1. Asymptomatic patients
a. The disease process of AS is characterized by a long latent phase, during which the patient has no symptoms. This period is associated with nearnormal survival. The risk of sudden cardiac death in asymptomatic patients with critical AS is < 2% per year.
b. Although the underlying cause helps predict the age of symptom onset, there is marked individual variability in the length of the latent period and the subsequent rate of progression of disease. In general, among asymptomatic valvular AS patients, the mean aortic valve gradient rises by 7 mm Hg/y and the aortic valve area (AVA) decreases by 0.1 cm2/y.
c. Because of the variable rate of disease progression, all patients with AS should be advised to report the onset of any symptoms to their physician and should be followed clinically and with Doppler echocardiography with increasing frequency as the lesion progresses.
d. Once the valve becomes severely narrow, as evidenced by a peak Doppler velocity of 4 m/s across it, the likelihood of developing symptoms or requiring surgical intervention over the following 2 years is very high. This likelihood is increased if the valve is heavily calcified.
2. Symptomatic patients.
When symptoms of AS develop, the survival rate decreases markedly, unless aortic valve replacement (AVR) is performed.
a. Patients with angina have a 50%, 5-year survival rate without surgical intervention. Those with syncope have a 50%, 3-year survival rate without surgical intervention. Patients with heart failure have a mean survival time of < 2 years if treated medically.
b. In patients with severe, symptomatic AS, sudden cardiac death can occur in the setting of hypotension or arrhythmia due to ischemia, LVH, or impaired LV function.
c. Signs and symptoms of severe AS might be subtle in some patients. Information concerning activity levels from family members may be useful in this situation, as may a symptom-limited stress echocardiogram. Exercise stress testing is absolutely contraindicated in the setting of definite symptoms.
E. Clinical manifestations
1. Signs and symptoms.
The onset of symptoms usually indicates progression to severe AS and heralds the need for surgical evaluation.
a. Angina. Patients with severe AS can experience ischemia from myocardial supply—demand mismatch due to high LV diastolic pressures, decreased myocardial perfusion, and increased wall stress. Angina can also result from underlying coronary artery disease (CAD). CAD is common among patients with severe AS. It occurs in 40% to 80% of patients with angina and in 25% of patients without angina.
b. Syncope. Because of fixed LVOT obstruction, patients with severe AS are unable to augment their cardiac output under conditions of low systemic vascular resistance (SVR) (i.e., induced by certain medications or vasovagal reactions). The ensuing hypotension can cause presyncope, syncope, or even cardiovascular collapse and death. Syncope can also result from atrial or ventricular arrhythmias, abnormal baroreceptor function, or abnormal vasodepressor responses induced by LV pressure overload.
c. Heart failure symptoms, such as exertional dyspnea, orthopnea, or paroxysmal nocturnal dyspnea, and fatigue can result from LV systolic or diastolic dysfunction.
2. Physical findings
a. Arterial examination. A hallmark finding in AS is a diminished and delayed carotid upstroke, pulsus parvus et tardus. However, elderly patients
with noncompliant vessels or patients with concomitant AR may often maintain a normal carotid pulsation, despite severe AS. These findings are rare with obstruction above or below the valve. It is classically thought that severe AS is not associated with hypertension, as the narrowed valve limits the flow into the arterial system and thus gives rise to a narrowed pulse pressure and relative hypotension. In fact, in the elderly, hypertension and severe AS may often coexist, likely as a result of impaired elasticity of the aortic walls, and the finding of arterial hypertension does not preclude significant associated AS.
with noncompliant vessels or patients with concomitant AR may often maintain a normal carotid pulsation, despite severe AS. These findings are rare with obstruction above or below the valve. It is classically thought that severe AS is not associated with hypertension, as the narrowed valve limits the flow into the arterial system and thus gives rise to a narrowed pulse pressure and relative hypotension. In fact, in the elderly, hypertension and severe AS may often coexist, likely as a result of impaired elasticity of the aortic walls, and the finding of arterial hypertension does not preclude significant associated AS.
b. Palpation. With LVH and normal LV cavity dimensions, the apical impulse is usually nondisplaced, diffuse, and sustained. However, the apical impulse may later be displaced when there is LV systolic dysfunction. A double apical impulse represents a palpable a wave or S4, caused by a noncompliant left ventricle. A systolic thrill may be palpable in the second right intercostal space.
c. Auscultation. The main auscultatory findings are shown in Figure 15.2.
(1) The typical murmur of AS is a systolic ejection murmur heard at the right upper sternal border that radiates to the neck. With a mobile bicuspid valve, an aortic opening sound may precede the murmur. As the severity of stenosis increases, the murmur becomes longer and peaks later in systole. The intensity of the murmur does not necessarily correspond to the severity of AS. S1 is usually normal in AS. As the AS becomes more severe, the aortic component of S2 diminishes and eventually disappears, resulting in a soft, single S2. Often, with severe AS, S2 is paradoxically split because of the prolonged ejection duration through the severely narrowed valve. S3 is indicative of poor LV systolic function. An S4 is common because of reduced LV compliance.
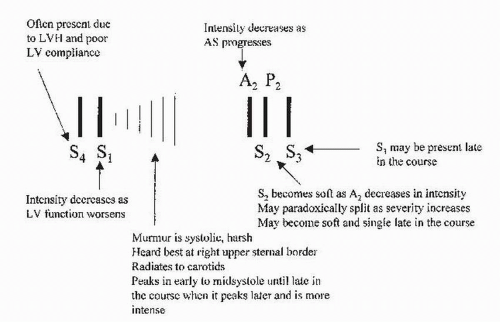 FIGURE 15.2 Auscultatory findings in aortic stenosis. LVH, left ventricular hypertrophy; LV, left ventricular. |
TABLE 15.1 Physical Findings and Maneuvers Useful in Distinguishing Various Forms of Left Ventricle Outflow Tract Obstruction | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
(2) Careful examination for other murmurs should be performed. AS is often accompanied by AR. Maneuvers performed during the physical examination can help differentiate different types of LV outflow obstruction, whether this is at, below, or above the valve. These are summarized in Table 15.1.
3. Diagnostic testing
a. The typical electrocardiogram (ECG) of a patient with isolated severe AS usually demonstrates left atrial abnormality (80% of cases) and LVH (85% of cases).
b. Chest radiography can be entirely normal, even in patients with critical AS. The cardiac silhouette may become boot-shaped because of concentric LVH. Cardiomegaly may be identified if there is LV dysfunction or coexisting AR. Aortic valve and root calcification can be seen in adults with severe calcific, degenerative AS. Poststenotic dilation of the ascending aorta may be evident.
4. Severity of AS.
The severity of AS is currently graded by ACC/AHA valve guidelines as follows:
Mild: valve area > 1.5 cm2, mean gradient < 25 mm Hg, or jet velocity < 3.0 m/s
Moderate: valve area 1.0 to 1.5 cm2, mean gradient 25 to 40 mm Hg, or jet velocity 3.0 to 4.0 m/s
Severe: valve area < 1.0 cm2, mean gradient > 40 mm Hg, or jet velocity > 4.0 m/s
The normal aortic valve opens to 3 to 4 cm2.
5. Echocardiography
a. Transthoracic Doppler echocardiography is the test of choice per ACC/AHA guidelines to establish the diagnosis of AS, to determine the cause and location, and to assess its severity. It should be performed when the diagnosis of AS is first suspected and information such as LV wall thickness, size, and function should also be obtained. After the diagnosis is established, patients should have frequent, regular clinical follow-up examinations to look for the development of symptoms. Echocardiographic follow-up can be tailored to the severity of disease: at least annually for severe AS and more frequently
as the severity increases. Development of new symptoms and signs should quickly prompt a repeat evaluation.
as the severity increases. Development of new symptoms and signs should quickly prompt a repeat evaluation.
(1) The parasternal long-axis views, two dimensional and M-mode, provide valuable information for determining the mechanism and severity of AS. In this view, the coaptation line of the aortic valve is normally centered within the LVOT in a trileaflet valve. The leaflets of a bicuspid valve often have an eccentric closure line, typically posterior to the midline. Systolic leaflet doming can be seen in congenital AS and rheumatic AS. The degree of LVH, LV chamber enlargement, or left atrial enlargement can be quantitated using two-dimensional and M-mode imaging. The LVOT diameter used in the continuity equation is measured in the twodimensional, parasternal long-axis view.
Subaortic stenosis and supravalvular AS may also be detected in this view. Subaortic stenosis may be evident as a membrane below the aortic valve with normal motion of the valve. Pulsed Doppler may indicate that the obstruction is occurring below the valve and 2 dimensional echocardiogram often shows AR due to the turbulent jet hitting aortic valve leaflets and causing leaflet scarring and impaired coaptation. In supravalvular AS, narrowing above the valve is evident on imaging and with Doppler.
(2) The parasternal short-axis view is the most useful view for establishing the cause of congenital AS. The number of commissures and the shape of the valve orifice should be assessed (Fig. 15.3). Trileaflet valves open
as a triangle, whereas an elliptical opening suggests a bicuspid valve. In a unicuspid valve, the opening is elliptical but occurs across a radius rather than the diameter of the valve.
as a triangle, whereas an elliptical opening suggests a bicuspid valve. In a unicuspid valve, the opening is elliptical but occurs across a radius rather than the diameter of the valve.
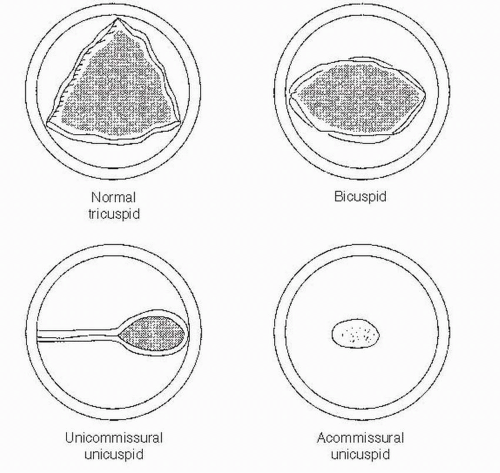 FIGURE 15.3 A schematic representation of parasternal short axis of a congenitally abnormal aortic valve. |
(3) The apical five-chamber and three-chamber views are well aligned with flow through the aortic valve. Continuous wave Doppler recordings across the aortic valve and pulsed wave Doppler flow in the LVOT proximal to the aortic valve are recorded in these views for the continuity equation.
(4) Continuous wave Doppler should be performed at multiple sites, including the suprasternal notch and the right sternal border, to ensure that the maximal velocity across the aortic valve is recorded. The dimensions of the ascending aorta should be measured, and coarctation in the descending aorta should be sought, especially in those with bicuspid aortic valves.
b. Transesophageal echocardiography (TEE). Planimetry of the aortic valve orifice is often possible with TEE and relates well to that measured by cardiac catheterization. Planimetry is difficult when the valve is extremely calcified. In bicuspid valve, the smallest area should be sought carefully, as the valve opening is not planar but rather forms a cone due to the doming of the valve as it opens. TEE is particularly useful for determining the morphologic features of the valve in congenital AS. TEE is often necessary to confirm the diagnosis of subaortic membrane and to differentiate it from hypertrophic cardiomyopathy or valvular AS.
c. Dobutamine echocardiography and stress echocardiography. Exercise testing in patients with asymptomatic AS may provide useful information such as exercise-induced symptoms or abnormal blood pressure response (ACC/AHA class IIb indication). Exercise testing is contraindicated (ACC/AHA class III indication) in symptomatic patients with AS. Dobutamine echocardiography may be useful in evaluating low-flow/low-gradient AS in patients with LV dysfunction (see Chapter 51 for further discussion).
6. Hemodynamic calculations
a. Doppler echocardiography is the standard modality used for the assessment of transvalvular pressure gradient and AVA.
(1) Simplified Bernoulli equation (ΔP = 4v2), in which P is pressure and v is peak velocity of flow across the aortic valve, is used to estimate the peak instantaneous gradient. The mean gradient across the valve can be measured by calculating the area under the Doppler envelope. The peak velocity of flow across the aortic valve should be measured in three areas: the LV apex, the right sternal border, and the suprasternal notch. The highest measured velocity is used to calculate the peak transvalvular gradient. When stenosis is present at two levels (i.e., in LVOT and at the valve), the gradient across the LVOT reflects the integrated effects of the obstruction at both levels. It is usually impossible with Doppler to precisely differentiate the contribution of each level of obstruction to the total. This may be inferred by the analysis of the images, by TEE, or by direct measurement at cardiac catheterization.
(2) Calculation of AVA is based on the continuity principle, which states that the flow of an incompressible fluid in a closed system must remain constant. Flow in a vessel is the product of the cross-sectional area (A) of the vessel and the velocity (V). Area is calculated as π R2 or π-D2/4 = 0.785-D2, where R is the radius of the vessel and D is the diameter. A schematic representation of the variables for calculating AVA is shown in Figure 15.4. The continuity equation for the aortic valve is as follows:
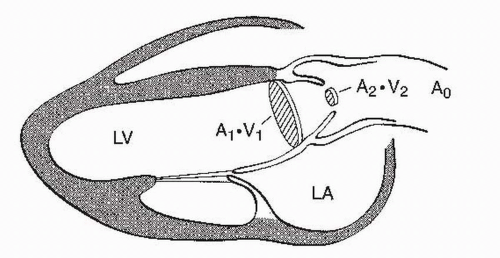 FIGURE 15.4 A schematic representation of parasternal long-axis view and the continuity principle. LV, left ventricle; LA, left atrium. |
In the above equation, VTI is the time-velocity integral. The continuity equation is valid only for valvular AS. It cannot be used to assess valve area when there are stenoses in series such as valvular and subvalvular narrowing occurring simultaneously.
(3) Care should be taken to avoid measuring postextrasystolic beats. If the patient is in atrial fibrillation, 10 consecutive beats should be measured and averaged for both velocity measurements.
(4) During evaluation of an aortic valve prosthesis, the standard continuity equation cannot be used. Instead, the velocity ratio or dimensionless index is used to estimate the severity of prosthetic stenosis. It is calculated by dividing the peak velocity in the LVOT by the peak velocity through the aortic valve. A dimensionless index of < 0.25 is generally accepted to represent severe stenosis. This is also useful if the LVOT diameter is difficult to ascertain.
b. Cardiac catheterization was once considered the gold standard for the quantification of AS but is now contraindicated (ACC/AHA class III indication) in the routine evaluation of a patient when echo-Doppler studies are adequate and congruent with clinical findings.
(1) Preoperative catheterization. Patients > 50 years of age, patients with angina, and patients with significant risk factors for CAD should undergo coronary cineangiography before aortic valve operations (ACC/AHA class I indication).
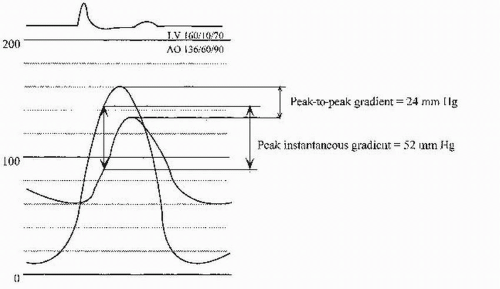
(2) Catheter-derived hemodynamic data are indicated (ACC/AHA class I indication) to further evaluate the severity of AS when the clinical and echocardiographic findings diverge. Because cardiac catheterization findings often differ from those of echocardiography, it is important to understand the differences in what is being measured. The mean gradients obtained during catheterization should be equivalent to the mean gradients obtained by echocardiography. These correlate well when performed expertly and simultaneously. The peak gradient measured during catheterization is the peak-to-peak gradient, which is lower than the peak instantaneous gradient obtained with echocardiography (Fig. 15.5). In the setting of reduced cardiac output of any cause, the aortic gradient may be much lower and may be < 20 mm Hg in severe LV dysfunction despite critical AS.
(3) The most precise measurement of transaortic valvular gradient is made with two different catheters (one in the LV cavity and the other in the ascending aorta) or with a double-lumen pigtail catheter. Because the two-catheter technique requires cannulation of both femoral arteries, another acceptable method of measuring the peak-to-peak gradient is catheter pullback from the left ventricle to the ascending aorta. A typical pressure tracing of simultaneous LV and aortic pressures is shown in Figure 15.5.
(4) Catheterization of the patient with severe AS should be performed with low-osmolar, nonionic contrast agents. These cause less hypotension due to peripheral arterial vasodilation, less bradycardia, less transient myocardial dysfunction, and less osmotic diuresis after the procedure. Left ventriculography should be avoided.
(5) The Gorlin formula is used to estimate AVA:
In the above equation, SEP is the systolic ejection period, defined as the time from aortic valve opening to closing (in seconds), and MVG is the mean valvular gradient (mm Hg). The Gorlin formula measures the true anatomic area of the aortic valve, as it has a correction factor (the discharge coefficient) to account for the difference of flow across the true anatomic valve versus the flow at the level of the vena contracta. Continuity equation measures the physiologic area (vena contracta) and as such is smaller than that measured by Gorlin.
An alternative measurement can be made by Hakki equation, which is a simplification of Gorlin formula, where the observation of heart rate × SEP approximates 1,000. This then simplifies the estimation of AVA:

F. Therapy
1. Medical therapy.
The mainstay of therapy for severe AS is surgical replacement of the aortic valve. Onset of symptoms in patients with severe AS is associated with a marked reduction in lifespan when treated medically, rather than surgically. Medical therapy alone is ineffective for severe symptomatic valvular AS.
a. Antibiotic prophylaxis. The updated 2007 AHA guideline on prevention of infective endocarditis has been extensively modified. Unless the patient has a valve prosthesis or prior history of infective endocarditis, antibiotic prophylaxis prior to dental procedures is no longer recommended in patients with valvular pathology.
b. Medical therapy in asymptomatic patients. During the asymptomatic phase, therapy is directed at primary prevention of CAD, maintenance of sinus rhythm, and blood pressure control.
c. Medical therapy in symptomatic patients. Medical therapies may be necessary in symptomatic patients with severe AS who are awaiting surgery or who are considered inoperable and require palliation. Therapy for heart failure is directed at relief of pulmonary congestion. This is usually achieved with cautious use of diuretics. Overly aggressive diuresis can cause hypotension if hypovolemia significantly impairs cardiac output by diminishing preload. Nitrates can also cause hypotension and syncope by reducing preload and should be avoided or used with extreme caution. Thus, the management of symptomatic patients with AS and CAD is difficult, and urgent surgery is the optimal treatment where feasible. Digoxin is used for symptom relief in the setting of impaired LV systolic function and volume overload, particularly if atrial fibrillation develops.
d. Vasodilator therapy has been relatively contraindicated in patients with AS, because lowering SVR in the setting of a fixed cardiac output may cause syncope, especially in the ambulatory setting. However, patients with severe heart failure and LV dysfunction with severe AS may benefit from the careful titration of intravenous nitroprusside in the intensive care unit with concomitant invasive arterial and pulmonary artery monitoring. One study suggests an improvement in hemodynamic indices with this approach. This therapy is used only as a bridge to definitive surgical therapy. Intraaortic balloon counterpulsation is another strategy that can be used while patients with LV dysfunction and severe AS in cardiogenic shock are worked up toward urgent surgery.
e. Treatment of hyperlipidemia.




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree