Fig. 15.1
ECG shows ST segment depression in I, aVL and V5-V6 leads
What Are the Possible Causes of Chest Pain in This Patient?
Acute coronary syndrome (unstable angina)
Valvular disease (aortic stenosis)
Acute pericardial disease
Chest wall pain
Lung diseases
The characteristics of chest pain were suggestive for typical angina, the patient was apyretic, the inflammatory biomarkers were negative, there weren’t clinical and instrumental signs of respiratory disease, and therefore, acute pericardial disease, chest wall pain, and lung diseases from differential diagnosis were excluded. The presence of systolic heart murmur and of ST–T downslope on rest ECG supported the hypothesis of ischemic heart disease or of aortic stenosis.
Echocardiography
Echocardiography was performed to better evaluate the aortic area (Fig. 15.2) and showed the following results: “Concentric left ventricular hypertrophy with normal left ventricle global and regional systolic function (EF 57 %). Severe dilatation of the left atrium (index volume 66 ml/m2). Pseudo-normal diastolic function. Normal right ventricle size and systolic function (TAPSE 17 mm). Mild dilatation of the right atrium. No pericardial effusion. Severely calcified tricuspid aortic valve with severe stenosis (peak velocity 4.3 m/s, medium gradient 36 mmHg, calculated aortic valve area 0.8 cm2, index calculated aortic valve area 0.43 cm2/m2). Mild mitral regurgitation. Mild tricuspid regurgitation with high pulmonary arterial pressure (40 mmHg).”
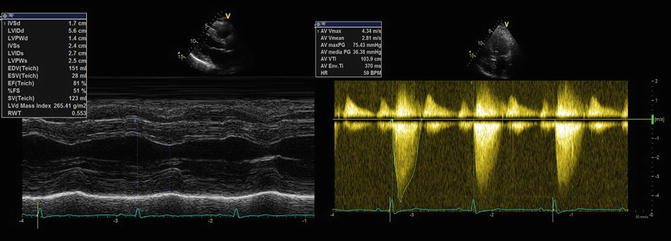

Fig. 15.2
Echocardiography. Concentric left ventricular hypertrophy (left side). Severe aortic valve stenosis with high trans-aortic valve gradient (right side)
Final Diagnosis
The final diagnosis is “severe aortic stenosis with effort angina in patient with known coronary artery disease.”
Symptomatic severe aortic stenosis has surgery correction indication; although the last PTCA was done in the previous 4 months, an invasive coronary angiography was performed because the angina symptoms show a LAD 70 % diameter stenosis (intrastent), severe stenosis of the first diagonal branch (D1) and second diagonal branch (D2), and a severe stenosis of the posterior descending artery (PDA) (Fig. 15.3a, b).
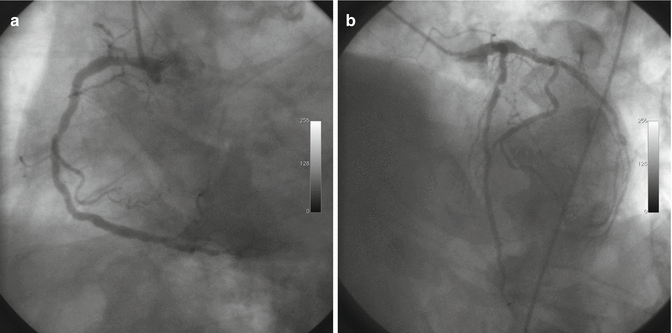

Fig. 15.3
Coronary angiography: right coronary (a); left coronary (b)
The patient was admitted to the cardio-surgery department to replace the aortic valve and for the heart to be revascularized through coronary artery bypass graft (CABG). A bioprosthetic aortic valve was implanted, and an artery graft with the left internal mammary artery to LAD and a saphenous vein graft to PDA were performed.
15.2 Aortic Stenosis
Epidemiology
Valvular aortic stenosis (AS) is the second most prevalent adult valve disease in the USA. The prevalence of AS increases with age: it occurs in 4 % of patients older than 75 years [1] and approximately in 2 % of population aged 65 years old.
Etiology
AS is a hereditary or acquired disease that causes a progressive obstruction to left ventricular outflow. Obstruction may be valvular, subvalvular, or supravalvular.
Valvular stenosis is the most common cause of aortic obstruction. Its etiology may be age related: patients younger than 20 usually have a congenital abnormality.
Patients aged 40–60 usually have a calcified bicuspid valve or a valve previously damaged by rheumatic heart disease.
The most common cause of AS in patients 70–80 years old is degenerative AS; this is an active process characterized by lipid accumulation, inflammation, and calcification of aortic valve cusps that provokes a valve degeneration [5–7].
Subvalvular AS occurs in less than 10 % of all patients with obstruction of left ventricular outflow and is frequently associated with aortic regurgitation due to valve damage. This kind of obstruction can be due to a subvalvular ridge or diffuse tunnellike narrowing of the entire outflow tract. If severe hypertrophy of the left ventricle is present, additional dynamic outflow obstruction from systolic anterior motion of the mitral valve may occur.
Supravalvular AS is an uncommon (less than 1 % of patients with aortic stenosis) congenital abnormality, consisting of narrowing of the ascending aorta (immediately over the aortic valve) secondary to a single stenosis or a long tubular lesion of the entire ascending aorta. This type of stenosis is often associated with some congenital abnormalities like coronary dysplasia, elfin facies, mental retardation, coarctation of the aorta, hypercalcemia, peripheral pulmonary stenosis, and Williams syndrome.
Physiopathology
AS represents a continuum disease: (1) an increase in afterload, (2) a decrease in systemic and coronary blood flow from obstruction, and (3) progressive hypertrophy. These mechanisms result in the classic symptom triad of dyspnea, angina, and syncope. Left ventricular cavity size and systolic function are initially maintained; in fact, the increase in left ventricular wall thickness acts as a compensatory mechanism to normalize wall stress. So at the beginning, hypertrophy is a beneficial adaptation.
However, this phenomenon may cause a reduced coronary flow reserve and oxygen supply–demand mismatch [8]. Hypertrophic hearts are more sensitive to diffuse subendocardial ischemic injury, which may result in both systolic and diastolic dysfunctions. The latter occurs from prolonged ventricular relaxation and decreased compliance and is caused by myocardial ischemia, a thick noncompliant left ventricle, and increased afterload [9, 10].
The so-called reversible phase is characterized by an obstruction progression to a higher level that provokes a decrease of left ventricle systolic function.
If afterload excess was continued, myocyte degeneration and fibrosis would occur (irreversible left ventricular systolic dysfunction). At this point, both the high afterload and the intrinsic myocardial disease significantly increase wall stress, and a vicious cycle of deterioration in ventricular function ensues.
A “death spiral” may occur: if systemic hypotension will occur (due to either drugs or a vasovagal reaction), perfusion of the coronary arteries may decrease; this increases the myocardial oxygen supply–demand mismatch and results in myocardial ischemia. The myocardial ischemia, in turn, reduces forward cardiac output, and aortic diastolic pressure decreases, further decreasing coronary perfusion pressure. Unless immediate steps are taken to increase perfusion pressure, progressive hemodynamic deterioration and death may occur.
Clinical Manifestation
The clinical presentation of AS varies. Many patients will remain asymptomatic for decades. The diagnosis of AS is usually made in these patients on the basis of a systolic murmur on auscultation and confirmed by echocardiography.
Symptoms usually consist of one or more of the classic triad of exertional dyspnea, angina, and syncope. The cause of syncope may include ventricular arrhythmias, a sudden decrease in systemic flow caused by the obstruction, or abnormal vasodepressor reflexes caused by the high left ventricular intracavitary pressure. Albeit rare, sudden death may be the initial manifestation of aortic stenosis.
Uncommonly, patients with end-stage AS and concomitant left ventricular dysfunction present with anasarca and cardiac cachexia.
The development of symptoms is a critical point in the natural history of patients with AS. In fact, after symptom onset, the average survival is 2–3 years.
Physical Examination
The physical examination of a patient with AS reveals classic, characteristic findings that are important for diagnosis and estimation of severity of this valvular disease.
The characteristics of the carotid upstroke are a reliable indicator of the severity of AS in most patients. There will be both parvus (small pulse) and tardus (slow upstroke) in patients with severe AS. These typical findings couldn’t be present on carotid palpation in older patients with a noncompliant vasculature. When there is a thrill felt in the right carotid artery but not in the left carotid artery, the diagnosis of supravalvular AS should be suspected. This phenomenon is due to the high-velocity jet of blood directed toward the innominate artery.
A sustained bifid left ventricular impulse indicates concomitant left ventricular hypertrophy. A systolic thrill, if present, indicates the presence of severe AS (mean gradient >50 mmHg).
Accurate auscultation is an essential component of evaluating patients with AS. An absent aortic component of the second heart sound indicates severe calcification of the aortic valve. A delayed murmur apex is also present in severe AS.
A fourth heart sound is frequently audible. The turbulence across the aortic valve always produces a systolic ejection murmur. It is not necessarily the intensity but the timing of the murmur that determines the severity of AS. In patients with mild AS, the murmur is characterized by an early peak, and the duration ends before the second heart sound. As AS becomes more severe, the peak intensity of the murmur occurs in mid-to-late systole, and the murmur extends into the second heart sound. The murmur of AS must be differentiated from that of hypertrophic obstructive cardiomyopathy or mitral regurgitation due to a flail posterior leaflet. A Valsalva maneuver may be of help in this case.
So we can say that severe AS is characterized by (1) a dampened upstroke of the carotid artery, (2) a sustained bifid left ventricular impulse, (3) an absent A2, and (4) a late-peaking systolic ejection murmur.
Electrocardiography
The electrocardiography usually shows sinus rhythm with left ventricular hypertrophy and left atrial enlargement. An ST interval depression, a T wave inversion, or a left bundle block can be present.
Chest Radiography
Chest radiography may demonstrate enlargement of the ascending aorta and a left ventricular predominance. Aortic calcification is frequently seen on lateral chest radiographs (i.e., porcelain aorta). This find implies high risk for operation [11].
How to Assess Aortic Stenosis
Echocardiography
In addition to physical examination, electrocardiography, and chest radiography, various imaging modalities may be used to confirm the presence of aortic stenosis.
Transthoracic echocardiography is the gold standard modality for initial diagnosis and subsequent evaluation of aortic stenosis; with this ultrasound technology, the physician can also determine the level of obstruction (supravalvular, valvular, or subvalvular), the number of aortic cusps, and the degree of cusp fusion.
The severity of aortic stenosis cannot be determined by visualization of valve motion alone, and Doppler echocardiography must be used to further assess the severity of aortic valve disease [12].
The main hemodynamic parameters recommended for clinical evaluation of AS severity with transthoracic echocardiography are [13]:
1.
AS jet velocity
2.
Mean transaortic gradient
3.
Valve area by continuity equation
The antegrade systolic velocity across the narrowed aortic valve, or aortic jet velocity, is measured using continuous-wave (CW) Doppler (CWD) ultrasound [14–16].
AS jet velocity is defined as the highest velocity signal obtained from any window after a careful examination. Color Doppler is also helpful to avoid recording the CWD signal of an eccentric mitral regurgitation (MR) jet.
A smooth velocity curve with a dense outer edge and clear maximum velocity should be recorded. The maximum velocity is measured at the outer edge of the dark signal. To get the velocity–time integral (VTI) for the continuity equation and the mean gradient, it is necessary to draw the outer edge of the dark “envelope” of the velocity curve (Fig. 15.4) [13].
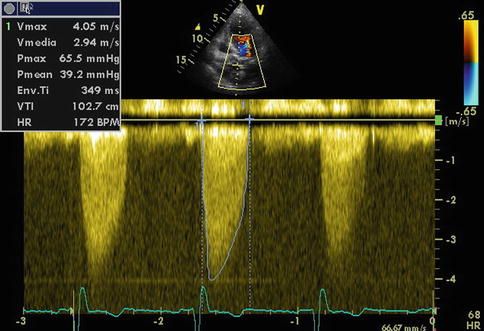

Fig. 15.4
Continuous-wave Doppler of aortic stenosis
To distinguish the level and severity of obstruction, the shape of the CW Doppler velocity is helpful; the maximum velocity occurs later in systole, and the curve is more rounded in shape with more severe obstruction [13].
To determine the fixity or the dynamism of obstruction, the shape of the CWD velocity curve also can be helpful. A characteristic late-peaking velocity curve is shown by a dynamic obstruction, with a concave upward curve in early systole (Fig. 15.5), in a patient with severe anemia.
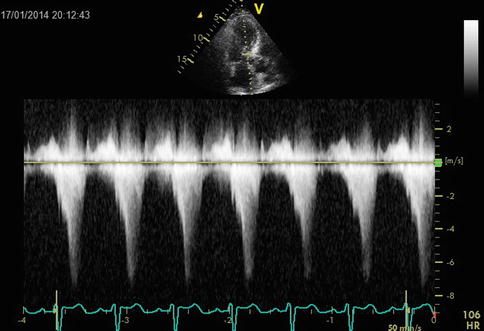

Fig. 15.5
An example of dynamic outflow obstruction. Note the different shapes of the velocity curves and the later maximum velocity with dynamic obstruction
Transaortic pressure gradient (ΔP) is calculated from velocity (v) using the Bernoulli equation as
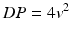 The maximum gradient is calculated from maximum velocity
The maximum gradient is calculated from maximum velocity
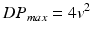 and the mean gradient is calculated by averaging the instantaneous gradients over the ejection period [13].
and the mean gradient is calculated by averaging the instantaneous gradients over the ejection period [13].
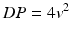
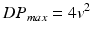
The aortic valve area can be calculated by Doppler echocardiography using the continuity equation.
From the parasternal long-axis view, the operator must get the left ventricular outflow tract (LVOT) diameter and convert it to the LVOT area. The LVOT velocity is obtained and traced to derive the time–velocity integral (TVI) from an apical approach with pulsed-wave Doppler.
Continuity equation
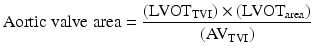 Significant errors in the area calculation may be caused by small errors in diameter measurement; for this reason the calculation of aortic valve area with continuity equation does require a skilled echocardiographist to ensure accurate measurement of the LVOT diameter.
Significant errors in the area calculation may be caused by small errors in diameter measurement; for this reason the calculation of aortic valve area with continuity equation does require a skilled echocardiographist to ensure accurate measurement of the LVOT diameter.
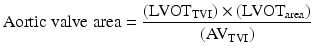
When LVOT diameter is squared for calculation of cross-sectional area, it becomes the greatest potential source of error in the continuity equation [13].
To reduce the errors associated with the measurements of the LVOT, this ratio can be used:
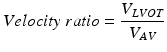 This dimensionless velocity ratio expresses the size of the valvular effective area as a proportion of the cross-sectional area of the LVOT [13]. In the absence of valve stenosis, the velocity ratio approaches 1. If the velocity ratio is 0.25 or less, severe stenosis is present [17].
This dimensionless velocity ratio expresses the size of the valvular effective area as a proportion of the cross-sectional area of the LVOT [13]. In the absence of valve stenosis, the velocity ratio approaches 1. If the velocity ratio is 0.25 or less, severe stenosis is present [17].
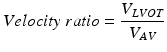
Aortic valve area planimetry may be an acceptable alternative when Doppler estimation of flow velocities is unreliable. When valve calcification causes shadows or reverberations limiting identification of the orifice, planimetry may be inaccurate.
Severe aortic stenosis can be diagnosed if a patient has these features:
Clinical findings consistent with severe aortic stenosis
A mean gradient greater than 40 mmHg
AVA less than 1.0 cm2 (<0.6 cm2/m2 when indexed for body surface area)
Recommendations for classification of AS severity are listed in Table 15.1.
Table 15.1
Recommendations for classification of AS severity
Aortic sclerosis | Mild | Moderate | Severe | |
|---|---|---|---|---|
Aortic jet velocity (m/s) | ≤2.5 | 2.6–2.9 | 3.0–4.0 | >4.0 |
Mean gradient (mmHg) | – | <20 (<30a) | 20–40b (30–50a) | >40b (>50a) |
AVA (cm2) | – | >1.5 | 1.5–1.0 | <1.0 |
Index AVA (cm2/m2) | – | >0.85 | 0.85–0.6 | <0.6 |
Velocity ratio | – | >0.50 | 0.50–0.25 | <0.25 |
Low-Flow, Low-Gradient Aortic Stenosis with Low Left Ventricular Ejection Fraction (LVEF)
Low LVEF, LF-LG severe AS is characterized by the combination of these features:
Aortic valve effective orifice area (EOA) <1.0 cm2 or <0.6 cm2/m2 when indexed for body surface area
Low mean transvalvular gradient (i.e., <40 mmHg)
Low LVEF (<40 %), causing an LF state [18]
The diagnostic problem in this kind of pathology is to distinguish true severe from pseudosevere aortic stenosis. In true severe AS, the LV dysfunction is a secondary or concomitant phenomenon, while the primary culprit is deemed to be the valve disease. Instead, the main factor in pseudosevere AS is deemed to be myocardial disease, and AS severity is overestimated due to incomplete opening of the valve in relation to the LF state. Distinction between these two entities is essential: patients with true severe AS generally will benefit from aortic valve replacement (AVR), whereas those with pseudosevere AS may not benefit [18].
Dobutamine infusion is helpful in differentiating pseudosevere from true severe AS.
True severe AS is characterized by little or absent increase in effective orifice area and an increase in gradient that is congruent with the relative increase in flow; pseudosevere AS shows an increase in effective orifice area (EOA) and relatively little increase in gradient in response to increasing flow [18].
Some parameters and criteria have been proposed to identify patients with pseudosevere AS during dobutamine stress echocardiography:




Peak stress mean gradient <30 or <40 mmHg (depending on studies)< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


