VALVULAR HEART DISEASE
CHAPTER

Aortic and Pulmonary Valve Disease
NORMAL AORTIC VALVE ANATOMY
Normal aortic valves are tricuspid—with right, left, and noncoronary cusps—and have a valve area of 2 to 3 cm2. However, congenital variations in this anatomy are relatively common, particularly bicuspid valves. Most commonly, bicuspid valves result from fusion of the right and left coronary cusps, although any two cusps may be fused. More rare are unicuspid and quadricuspid valves, with one and four cusps, respectively (Fig. 33.1). Although some congenitally abnormal valves function normally and are clinically silent, they more frequently result in symptomatic aortic stenosis (AS) or aortic insufficiency (AI) by middle age.
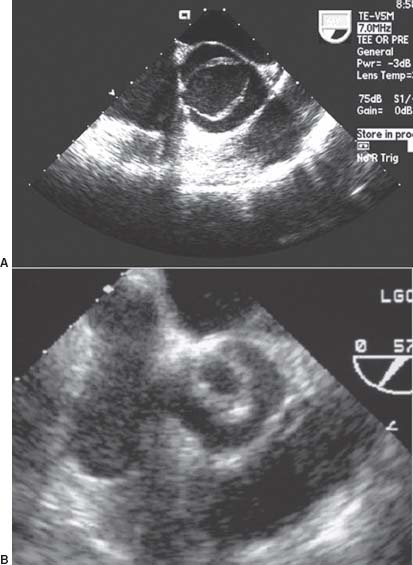
FIGURE 33.1 Transesophageal echocardiography short-axis images of bicuspid aortic valve (A) and unicuspid valve(B)
AORTIC STENOSIS
AS is one of the most frequent valve pathologies encountered in clinical cardiology. The etiology can be varied and may include subvalvular, valvular, or supravalvular lesions, but the pathophysiologic and hemodynamic responses to fixed outflow obstruction are usually predictable.
Pathophysiology of Aortic Stenosis
AS, regardless of degree, creates a pressure overload on the left ventricle (LV). Over time, the ventricle develops a compensatory concentric hypertrophy, which allows LV wall stress, or afterload, to remain normal, despite increased systolic pressures. This relationship is expressed by the law of Laplace, which states that wall stress is proportional to the chamber radius divided by its thickness. Thus, in compensated AS, left ventricular hypertrophy (LVH) functions to normalize after load and helps to maintain normal LV contractile function. The hypertrophy does, however, lead to increased LV mass and end-diastolic pressures, which in turn may precipitate diastolic heart dysfunction and myocardial ischemia. The degree of hypertrophy may vary dramatically among individuals, and gender differences have also been noted. Classically, women develop more hypertrophy with a small-to-normal LV cavity size, whereas men develop a lesser degree of hypertrophy, a dilated LV cavity, and earlier systolic dysfunction.1 Ultimately, compensatory mechanisms fail and patients develop symptoms due to progressive diastolic dysfunction, systolic dysfunction, compromised cardiac output, or myocardial ischemia.
Etiologies of Aortic Stenosis
There are multiple etiologies of AS, the most common of which are discussed below.
Degenerative AS is the most common etiology of AS in the United States. Once believed to be a passive process of calcification due to years of “wear and tear,” degenerative AS is now understood to be a dynamic process involving a robust inflammatory response of macrophages, T cells, and fibroblasts. The exact precipitants for AS are not clear, but observational evidence suggests the process may share some risk factors with atherosclerosis. In retrospective studies, statins have attenuated progression of AS2,3; however, no prospective studies have shown that medical therapy is effective in delaying progression of AS.
Rheumatic heart disease is a common cause of AS in less developed countries, but its incidence in developing countries has declined over the past 30 years.
Congenital valve disease may result in bicuspid or unicuspid valves, which are a frequent cause of symptomatic AS in younger patients (see Fig. 33.1). Bicuspid valves (BAV) have a prevalence of 1% to 2% in the population and are associated with other congenital abnormalities (especially coarctation) in 20% of cases. Up to 80% of patients with coarctation have BAV. Bicuspid valves are also associated with aortic root dilatation and an aortopathy that resembles cystic medial necrosis. Unicuspid valves are inherently stenotic and usually cause symptoms by the third decade of life. Like bicuspid valves, unicuspid valves also are associated with an aortopathy.
Radiation heart disease may occur in patients who have a history of mediastinal radiation as treatment for lymphoma, breast, or esophageal cancers. The risk of valve disease is increased in patients who have received >30 Gy of radiation and generally presents 15 to 20 years after exposure. There is a greater tendency toward aortic valve disease, followed by mitral and then tricuspid valve disease. Due to this distribution of valve involvement, it is thought that flow may play a role in the valvular disease. Usually, radiation-associated aortic disease is mixed stenosis and regurgitation. Subvalvular AS is a rare form of AS and may be due to a tunnel of muscular tissue or a discrete band or membrane. Sub-valvular stenosis should be suspected in any patient who has symptoms of AS or high LV outflow velocities on echo cardiography but whose aortic valve is structurally normal. Sub-valvular stenosis also presents as a component of the Shone complex: multiple left-sided heart obstructions, including supravalvular mitral stenosis, parachute mitral valve, subvalvular AS, BAV, and aortic coarctation. Additionally, subaortic stenosis may be associated with a patent ductus and ventricular septal defects (VSDs). Over time, the jet from subvalvular stenosis will damage the native aortic valve and will lead to AI. For this reason, early surgical repair of asymptomatic subvalvular stenosis is often recommended. Supravalvular AS is a rare variant of AS that is classically associated with Williams syndrome (child-like facies, peripheral pulmonary stenosis, hypercalcemia) and familial dyslipidemias. A mutation in the gene for elastin has been linked to Williams syndrome. Other cardiovascular associations of supravalvular stenosis include coarctation of the thoracic or abdominal aorta and renal artery stenosis.
Clinical Findings in Aortic Stenosis
The history of patients with AS varies with the etiology of the stenosis. Patients with rheumatic heart disease or bicuspid aortic valves frequently have a long history of a heart murmur. They also are more likely to present with symptomatic disease at a younger age. In contrast, patients with degenerative AS usually are older, in their seventh or eighth decade, and may present with symptoms without prior knowledge of aortic valve pathology.
The symptoms of AS are most frequently the direct result of the heart’s compensatory changes. Initially, patients develop diastolic heart dysfunction, which often manifests as exertional dyspnea. With stress, patients with AS may become significantly symptomatic because their cardiac output cannot augment adequately and left ventricular end-diastolic pressure (LVFDP) markedly increases. Dyspnea and early fatigability result. As the AS progresses, the classic symptoms of angina, syncope, and heart failure develop. This triad of symptoms has been well studied and allows a rough estimate of disease severity and prognosis: if untreated, survival in patients with angina approximates 5 years, with syncope is 3 years, and with heart failure is <2 years.
Angina is very common in severe AS and may be due to concomitant coronary disease, demand ischemia, or both. Interestingly, up to 50% of patients with angina and severe AS have no obstructive coronary disease. The angina is usually typical substernal pain, worsened with exertion or stress, and relieved with rest. Anginal equivalents, such as dyspnea on exertion, are also common. Syncope or presyncope most often results from exertional cerebral hypoperfusion; with exercise, the systemic arterial tree vasodilates, but the cardiac output remains relatively fixed. Arrhythmias may also precipitate syncope, especially atrial fibrillation or ventricular tachycardia. Congestive heart failure (CHF) symptoms such as pulmonary edema, paroxysmal nocturnal dyspnea, and orthopnea are late findings in AS and signify advanced disease with very poor prognosis if untreated.
Less common manifestations of AS include cardiac cachexia in very advanced cases and gastrointestinal bleeds from atriovenous (AV) malformations (Heyde Syndrome). Cardiac cachexia and debilitation result from a profound, longstanding low-output state. The mechanism for gastrointestinal bleeding from arteriovenous malformations is presumed to be destruction of large multimers of von Willebrand factor as they are sheared through the aortic valve. These larger multimers are apparently critical to the initial phases of hemostasis.
The Physical Exam
Vascular Findings The carotid pulsations in patients with severe AS are characterized by a delayed and weakened upstroke, the pulsus parvus et tardus. In long-standing critical AS, the peripheral pulses also may be weak, and signs of poor perfusion may be present.
Cardiac Findings On palpation, one may feel a systolic thrill in cases of severe AS. The apex may be laterally displaced if the heart has begun to dilate. The cardiac exam in AS is notable for a crescendo-decrescendo murmur, heard best in the right upper sternal border (RUSB) and radiating to the carotids. Occasionally, the murmur may instead radiate to the apex and mimic mitral regurgitation (MR); this is known as the Gallavardin phenomenon. As AS progresses, the murmur peaks increasingly later in systole until S2 is obliterated, suggesting severe disease. The grade of the murmur correlates with severity of the stenosis, and the presence of a thrill (Grade IV/VI) suggests critical stenosis. An S4 is also frequently appreciated. An ejection click suggests the presence of a bicuspid aortic valve.
The physical exam may be useful in differentiating valvular AS from hypertrophic cardiomyopathy (HCM) and subaortic stenosis. In HCM, the carotid pulsation is on time and is bifid, with a two-component, “spike and dome” contour. This is caused by the presystolic closure of the aortic valve. The point of maximal cardiac impulse (PMI) pulsation in HCM patients classically has three components, which correspond to atrial filling and the two components of systolic ejection. The murmur in HCM can be differentiated from AS by several maneuvers. Decreasing either preload or afterload will accentuate the murmur of HCM, but will soften the murmur of valvular AS. Thus, a Valsalva maneuver or arising from squatting to standing will accentuate a HCM murmur, but will decrease the murmur of AS. Amyl nitrate will similarly decrease afterload and preload, resulting in marked increase in the HCM murmur.
The murmur of subvalvular stenosis resembles valvular AS. Clues that the murmur might be due to subvalvular stenosis include a younger patient age, the presence of AI, and the absence of an ejection click. In supravalvular AS, blood flow preferentially is directed into the innominate artery, so the murmur of supravalvular stenosis classically radiates to the right neck and subclavian and may be associated with a thrill over the right carotid. The blood pressure in the right arm may be slightly higher than in the left. Careful auscultation of the lung fields may reveal murmurs associated with peripheral pulmonary stenosis.
Key Diagnostic Studies
Electrocardiogram The electrocardiogram (ECG) in patients with severe AS may show LVH with concomitant strain pattern, left atrial (LA) abnormality, or interventricular conduction delay. Transient third-degree heart block has been described, and has been ascribed to aortic annular calcification impinging on the AV nodal conduction system.
Chest X-Ray The chest x-ray (CXR) is often normal in patients with AS, especially because LVH frequently is unaccompanied by dilation early in the disease. With advanced disease, LVH and enlargement, aortic dilation, and aortic valvular calcification may be appreciated.
Transthoracic Echocardiogram Echocardiography has become the gold standard for diagnosis and quantification of aortic valve disease. Key data that are attained from a transthoracic echocardiogram (TTF) assessment include the following.
LV Size and Systolic Function. Systolic function is usually normal until late in the disease. The LV will show variable hypertrophy, with overall normal size.
Diastolic Function. Early in the disease process, LV compliance decreases and the atrial component of diastolic filling becomes increasingly prominent. Over time, LA pressure rises, and ultimately, patients with long-standing AS may develop restrictive diastolic filling patterns.
Assessing Aortic Valve Morphology. Echocardiography is paramount in identifying the etiology of AS. Standard transthoracic images usually can identify bicuspid or unicuspid valves, can suggest a rheumatic etiology, or can quantify the degree of valvular calcification.
Assessing the Severity of AS. There are several methods to estimate the severity of AS. Multiple methods should be used in each patient to ensure accurate data.
Jet velocity: Peak aortic valve jet velocity provides a rough measure of valve severity and also provides a measure of prognosis. A normal outflow velocity is approximately 1 m/s. Studies have suggested that asymptomatic patients with outflow gradients in excess of 4 m/s will most likely develop symptoms within 2 years.4
Valve gradients: Peak transaortic valve gradients can be estimated using the jet velocity and the modified Bernoulli equation: peak gradient = 4v2, where v is the peak velocity across the valve. Mean gradients are calculated using the velocity time integral (VTI).
Aortic valve area (AVA): AVA most frequently is estimated using planimetry on a parasternal short-axis image of the aortic valve or by using the continuity equation (Fig. 33.2).
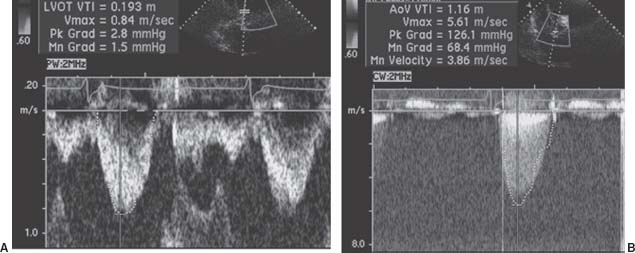
FIGURE 33.2 Pulse-wave Doppler flow through the LVOT (A) and continuous-wave Doppler flow through the aortic valve (B). The continuous-wave flow allows simple calculation of the peak transaortic gradient: peak = 4v2, where v is equal to the maximum flow across the aortic valve. In this case, v = 5.6 m/s, and the peak gradient is given as 126 mm Hg. The AVA can be calculated from the continuity equation, ALVOT(VTI)LVot = AAV(VTI)AV, where A is area and VTI is the velocity time integral, or the flow velocity integrated over the systolic ejection period. In this example, assuming the LVOT diameter is 2 cm,
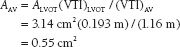
The dimensionless index: The dimensionless index refers to the ratio of the left ventricular outflow VTI to the aortic valve VTI. This ratio allows for a quick, semiquantitative assessment of valve stenosis. An index <25% is consistent with severe stenosis.
Several caveats must be kept in mind when using echocardiography to assess the severity of AS. First, Doppler echocardiography can underestimate the peak AS gradient if the echo beam is not accurately aligned with the aortic outflow. Therefore, multiple echo windows must be assessed to find the highest transvalvular velocities. Additionally, the modified Bernoulli equation assumes a left ventricular outflow tract (LVOT) velocity of 1 m/s, which may not always be true. If the true LVOT velocity is >1 m/s, then the modified Bernoulli equation will overestimate stenosis severity. Finally, continuous-wave Doppler cannot assess stenoses in series, such as dynamic LVOT obstruction and valvular AS, or subvalvular and valvular AS. In such instances, the use of the continuity equation can be erroneous. Care must also be taken not to confuse the Doppler signals of AS and MR. Generally, MR velocity is 4 to 5 m/s, and the Doppler signal begins at the start of systole (i.e., no period of isovolumic contraction).
Invasive Assessment of the Aortic Stenosis
Echocardiography usually is sufficient to determine the severity of AS; however, invasive assessment of AS is sometimes necessary, particularly in cases in which clinical symptoms are not congruent with echo data (see American College of Cardiology/American Heart Association [ACC/AHA] Guidelines5). During right heart catheterization, Fick cardiac outputs are preferable to thermodilution because they are more reliable in low-output states. During left heart catheterization, simultaneously measured LV and ascending aortic pressures are ideal, although a pullback gradient may be used if the patient is in sinus rhythm. The femoral artery waveform should not be used to estimate aortic pressures. The AVA can be estimated with the Hakki equation: AVA = CO/√(peak or mean transvalvular gradient). A formal calculation can be done with the Gorlin equation (Fig. 33.3).
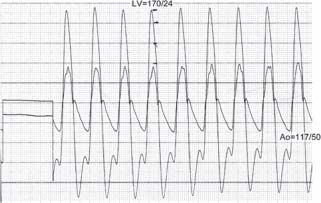
FIGURE 33.3 Simultaneous pressure recordings of the LV and aorta. Peak-to-peak gradient is approximately 53 mm Hg. If one knows the cardiac output (CO), the AVA can be estimated with the Hakki equation: AVA = CO/(peak gradient)1/2. Assume that the CO = 4 L/m. Then
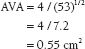
(Courtesy of D. Vivek.)
Invasive hemodynamic assessment measures a peak LV-to-peak aortic gradient across the aortic valve, which is invariably lower than the peak gradient on echocardiography. This is due to the fact that Doppler echocardiography measures the peak instantaneous velocity. The mean transvalvular gradients by echo and catheterization correlate well, however. Occasionally, Doppler gradients greatly exceed gradients on catheterization, which may be due to the phenomenon of pressure recovery. In effect, substantial turbulent flow of blood through the valve may cause the pressure in the aorta to be artificially low immediately distal to the valve. Several centimeters into the proximal aorta, however, laminar flow is restored, and pressure “recovers.” Doppler echocardiography detects the maximum pressure gradient between the LV and the proximal aorta. Pressure recovery usually is not an issue with native aortic valves but can be problematic especially with smaller prosthetic valves.
Classifications of Severity of Aortic Stenosis
Normal AVA: 2 to 3 cm2
Mild AS: AVA > 1.5 cm2, mean gradient <25 mm Hg
Moderate AS: 1.0 to 1.5 cm2, mean gradient 25 to 40 mm Hg
Severe AS: <1.0 cm2, mean gradient >40 mm Hg
Treatment of Patients with Aortic Stenosis
Asymptomatic Patients
Patients with AS who have no symptoms may be managed expectantly, as the risk of adverse events—for example, sudden death, cardiac death, or all-cause mortality—is very low in asymptomatic patients.6 Endocarditis prophylaxis is no longer indicated for patients with AS, though is reasonable (Class IIa) for patients with previous endocarditis or prosthetic material.7 Vasodilators should be used with extreme caution due to concerns of diminishing preload. The ACC guidelines provide a Class I indication for serial echocardiography every 3 to 5 years for patients with mild AS, and every 1 to 2 years for patients with moderate AS. For patients with severe AS, surveillance echocardiography is recommended on an annual basis, or even more frequently if clinically indicated.5 Stress echocardiography may be helpful in assessing patients with asymptomatic AS to evaluate functional capacity and assess for abnormal blood-pressure response or unrecognized symptoms (Class IIb indication). Patients with symptomatic AS should not have stress testing (Class III).
Patients with mild AS are encouraged to keep physically active and may participate in competitive sports. For patients with moderate AS, aerobic activity is permissible; however, competitive contact sports or heavy lifting is not advised. Patients with severe AS should not engage in strenuous activity or competitive sports.
Progression of AS is highly variable, but on average, valve area decreases approximately 0.1 cm2/y. Progression of AS has been associated with risk factors for coronary artery disease (CAD) (diabetes, hypercholesterolemia, hypertension) in observational studies, and thus treatments of these conditions may be important is AS therapy as well. A more rapid rate of change (>0.3 m/s/y increase in velocity)8 or a peak jet velocity >4 m/s suggest that patients have <2 years before symptoms will develop.4,8 Heavily calcified valves are also associated with a higher likelihood of symptomatic disease.
Any patient with severe AS should be aware that dyspnea, angina, or presyncope merits prompt evaluation. Symptoms of AS may be insidious, however, and patients may be unaware of a decline in functional capacity. In such cases, stress echocardiography can be useful to assess functional capacity, ventricular function, transvalvular gradients with stress, and the pulmonary artery (PA) pressure responses to stress. Such variables may alter the threshold to pursue aortic valve replacement (AVR).
Patients with Rheumatic Fever
Rheumatic fever is an important cause of both aortic and mitral valve disease. Its prevalence has decreased over the past 30 years, largely because of better diagnosis and treatment of group A streptococcal pharyngitis. Acute rheumatic fever is recognized as a serious complication of pharyngeal streptococcal infections, and is due to an autoimmune phenomenon triggered by group A streptococcal M proteins, which mimic cardiac myosin.
Primary prevention of rheumatic fever includes prompt diagnosis of group A streptococcal infections and treatment with appropriate antibiotics, usually a penicillin derivative or macrolide. Patients who develop acute rheumatic fever require long-term secondary prophylaxis, usually with monthly intramuscular injections of benzathine penicillin (Table 33.1).
TABLE
33.1 Recommendations for Secondary Prophylaxis of Rheumatic Fever
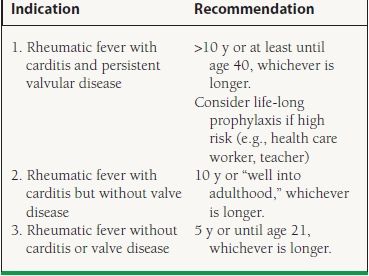
From Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused Update Incorporated Into the ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1-e142, with permission from Elsevier.
Symptomatic Patients
For patients with severe AS who develop symptoms, experience a decline in LV systolic function <50%, or are undergoing other open heart surgery (OHS), AVR is recommended as a Class I indication.5 The risk of AVR increases with patient age; however, because the prognosis for untreated, severe symptomatic AS is abysmal, chronologic age alone should not be used to exclude patients from valve surgery. Low ejection fraction, heart failure, renal failure, female gender, and atrial fibrillation also are adverse predictors in patients undergoing aortic valve surgery. Transcatheter aortic valve implantation (TAVI) has emerged as an attractive alternative procedure to surgical AVR, and initial results in patients unable to undergo surgery are promising.9
AVR is reasonable (Class IIa) for patients with moderate AS undergoing OHS for a different reason, and may be considered (Class IIb) for patients with asymptomatic severe AS and abnormal exercise testing or features concerning for rapid progression (age, calcification, and CAD). AVR may also be considered (Class IIb) for patients with asymptomatic and “extremely severe” AS (AVA <0.6 cm2, mean gradient >60 mm Hg, or jet velocity >5 m/s) if the operative mortality is <1% (Table 33.2).5
TABLE
33.2 Indications for Aortic Valve Surgery in Patients with Severe AS
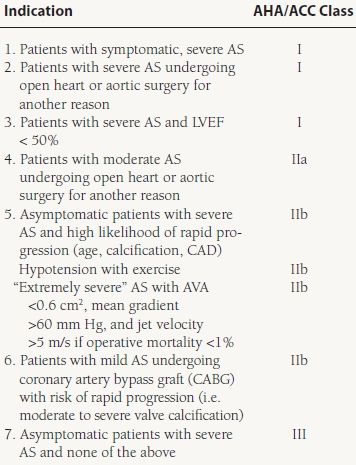
From Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused Update Incorporated Into the ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1-e142, with permission from Elsevier.



