Chapter 15
Anticoagulants in Venous and Arterial Disease
Sue Pavord and Amy Webster
Department of Haematology, University Hospital of Leicester NHS Trust, UK
Heparin
Heparins are naturally occurring sulphated glycosaminoglycans isolated from animal tissue, most commonly porcine intestine. They indirectly inhibit blood coagulation, through their effect on antithrombin. A specific five-sugar (pentasaccharide) sequence of the heparin molecule binds with high affinity to antithrombin, inducing conformational change and enhancing its inhibitory activity by some 2000-fold. The main effect is on thrombin and factor Xa, key factors in the coagulation cascade and development of the fibrin clot (Figure 15.1).
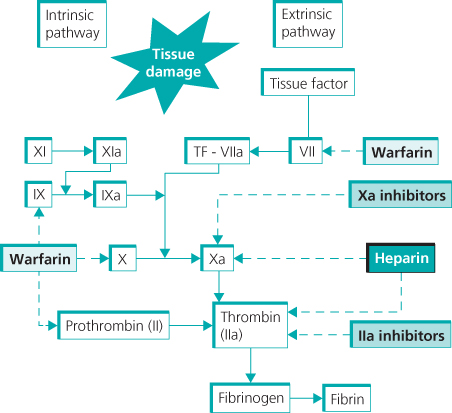
Figure 15.1 Coagulation system and sites of inhibition by different anticoagulants.
Heparin is widely used for therapeutic and prophylactic anticoagulation. It has a rapid onset of action and short half-life but can only be administered parenterally due to its rapid destruction by gut enzymes.
Unfractionated heparin (UFH)
The mean molecular weight of UFH (unfractionated heparin) is 15 000 Da, which allows it to bind to both antithrombin and thrombin, giving both anti-IIa and anti-Xa activity in roughly equal measure. It is given intravenously and requires a loading dose before continuous infusion. Doses are adjusted according to the activated partial thromboplastin time (APTT), which should be measured every 6 h until stable. The therapeutic APTT range depends on the sensitivity of the reagent used and needs to be determined locally. The required doses to achieve therapeutic anticoagulation can be variable due to the non-specific binding to plasma proteins.
The use of UFH in first-line treatment for VTE (venous thromboembolism) has largely been superseded by low molecular weight heparin (LMWH) and oral direct inhibitors (NOACS), although UFH is still useful for patients with renal impairment.
Low molecular weight heparin (LMWH)
LMWHs are produced by chemical or enzymatic cleavage of UFH. The action of LMWH is primarily on anti-Xa, as the smaller molecular size (3000–5000 Da) renders it unable to form a tertiary ternary heparin–thrombin–antithrombin complex required for the inactivation of thrombin (Figure 15.2).
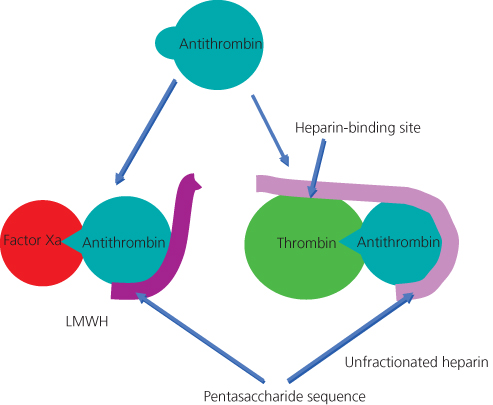
Figure 15.2 Heparin binding to antithrombin. Only heparins of more than 18 saccharide units can complex antithrombin to thrombin.
LMWH is given by subcutaneous injection and has more predictable pharmacokinetics, largely because of the lack of non-specific plasma protein binding. Dosing is weight dependent, and maybe once or twice daily, dependent on the preparation. Excretion is primarily renal and dosing adjustments may be required in renal impairment. Monitoring is not routinely required, but in cases of renal failure or extremes of weight, measurement of anti-Xa activity can be helpful. LMWH has been shown to be as effective as UFH in the treatment of VTE and allows for outpatient management in suitable patients. Other advantages of LMWH are shown in Table 15.1.
Table 15.1 Comparison of unfractionated and low molecular weight heparins.
| Unfractionated heparin | Low molecular weight heparin |
| Increased half-life with increased concentration of drug (range 30 min to 4 h) | Stable half-life, ∼4 h, and more predictable dose response |
| Non-specific protein binding | Reduced non-specific binding |
| <50% bioavailability subcutaneously (at low dosage) | >90% bioavailability subcutaneously |
| Monitoring required with APTT | No monitoring required (anti-Xa if occasionally needed) |
| Risk of heparin-induced thrombocytopenia | Lower risk of heparin-induced thrombocytopenia |
| Hepatic and renal elimination | Renal elimination |
| Risk of osteoporosis with prolonged therapy | Lower risk of osteoporosis |
Fondaparinux
Fondaparinux is a synthetic pentasaccharide, chemically related to the antithrombin-binding site of heparin. Like heparin, it has to be administered parenterally and is used for the prevention and treatment of VTE.
Vitamin K antagonists
Vitamin K is required as a cofactor for the conversion of glutamic acid to γ-carboxyglutamic acid in the synthesis of coagulation factors II, VII, IX and X (Figure 15.3) and the natural anticoagulants, proteins S and C. Vitamin K antagonists, of which warfarin is most commonly used, competitively inhibit this process. The peak pharmacological effect is at approximately 48 h post dose because of the time taken for degradation of existing clotting factors. Furthermore, the initial period can be associated with transient hypercoagulability due to the reduction in the natural anticoagulant proteins C and S, which have shorter half-lives than the procoagulant factors II, IX and X. This occurs despite a prolonged INR (international normalised ratio), which is largely determined by the fall in FVII. Therefore, on starting warfarin for treatment of VTE, concomitant LMWH should be given for at least 5 days.
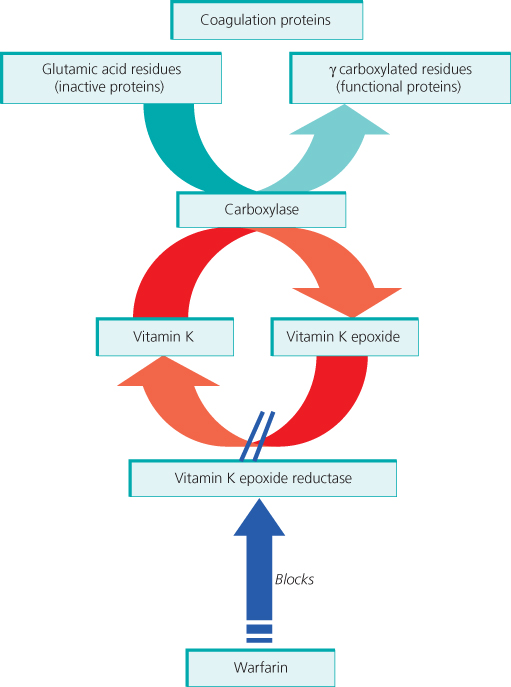
Figure 15.3 Warfarin inhibits vitamin K epoxide reductase, thus reducing functional coagulation protein synthesis.
Warfarin is orally bioavailable and rapidly absorbed in the gastrointestinal tract. It is metabolised by the hepatic cytochrome P450 system and is highly plasma protein bound, causing many pharmacological interactions and variation in half-life between patients. It, therefore, requires close monitoring and often minor dose changes to ensure stable anticoagulation. Factors influencing warfarin control are outlined in Box 15.1.
Warfarin is monitored using the prothrombin time (PT), which is expressed as the ratio of the PT of the patient to that of a pool of healthy donors. PT measurements have been standardised internationally by the use of an assigned international sensitivity index (ISI) to each thromboplastin reagent, which compares the reagent to a world standard. INR is then calculated as follows:
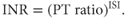
For most indications, including treatment of VTE, the target INR is 2.5 (range 2–3). If VTE recurs despite the INR being in the therapeutic range, the target should be increased to 3.5. Because the therapeutic range is narrow, there is a significant risk of over-anticoagulation and bleeding episodes are not infrequent. Table 15.2 indicates the increased risk of bleeding associated with high INRs.
Table 15.2 Risk of bleeding according to level of INR.
| INR | Relative bleeding risk |
| 2.0–2.9 | 4.8 |
| 3–4.4 | 9.5 |
| 4.5–6.9 | 40.5 |
| ≥7 | 200 |
Source: Palareti et al. (1996). Reproduced by permission of Elsevier.
Novel oral anticoagulants
Factor Xa inhibitors
Factor Xa inhibitors, such as rivaroxaban and apixaban, act by direct and selective inhibition of factor Xa (Figure 15.1). They are licensed for prevention of VTE following hip and knee arthroplasty and for stroke prevention in non-valvular atrial fibrillation (AF). Rivaroxaban is also licensed for treatment of acute VTE, with a recommended dose of 15 mg twice daily for the initial 3 weeks followed by 20 mg daily for completion of treatment. The majority of the drug undergoes hepatic metabolism and rivaroxaban is therefore contraindicated in moderate to severe hepatic dysfunction. Apixaban is likely to gain its licence in the near future, as data from a large randomised controlled study have confirmed non-inferiority to conventional therapy in treatment of acute VTE. Both provide a predictable anticoagulant effect, obviating the need for monitoring. Owing to significant renal clearance, they are contraindicated in patients with a creatinine clearance <15 ml/min.
Direct thrombin inhibitors
Dabigatran etexilate is a pro-drug that is converted to its active form, dabigatran, after oral administration. It acts by competitive and reversible inhibition of thrombin, preventing the conversion of fibrinogen to fibrin (Figure 15.1). Elimination is primarily renal and dose adjustments should be made in renal impairment. Currently, it is licensed for VTE prevention following hip or knee replacement surgery and for stroke prevention in non-valvular AF. A large randomised controlled study (RE-COVER) showed that it was as effective as warfarin, with a similar bleeding profile, in the treatment of acute VTE.
Anticoagulation for acute venous disease
Anticoagulation for the treatment of PE (pulmonary embolism) and DVT (deep vein thrombosis) is the same and has two roles: initially preventing extension and propagation of thrombosis and thereafter preventing a recurrent event. The 2012 NICE guidelines recommend LMWH or fondaparinux as first-line agents, with UFH as an alternative in patients with renal impairment (eGFR < 30 ml/min), significant bleeding risk or in patients with PEs who are haemodynamically unstable and may require thrombolytic therapy. Vitamin K antagonists should be started simultaneously and both continued until the INR is >2 for at least 24 h. Subsequent guidelines also offer the oral direct inhibitors (NOACs) as an alternative, based on the data in prospective randomised controlled trials.
Inferior vena cava (IVC) filters are only recommended as a temporary option in those patients who have a contraindication to anticoagulation, such as active bleeding, and, if fitted, these should be removed when patients are eligible to start treatment (Figure 15.4).
Duration of anticoagulation
Proximal DVT (popliteal vein and above) and PE require at least 3 months of therapeutic anticoagulation. The duration of required anticoagulation thereafter is dependent on a number of factors (Box 15.2) but most importantly, the circumstances around the thrombotic event and the presence or absence of any provoking transient risk factors. For example, a patient with below-knee DVT following surgery is likely to need only 6 weeks of anticoagulation compared to an individual with spontaneous PE who would be considered for lifelong treatment. Where there is a clear provoking factor, such as surgery, or a transient risk factor that has resolved, such as pregnancy or the combined contraceptive pill, longer term anticoagulation is not usually recommended.
Unprovoked proximal VTE or PE is associated with a significant risk of recurrence of >9%. The decision to continue long-term anticoagulation needs to be balanced against the bleeding risks of

Full access? Get Clinical Tree


