Trial
Inclusion criteria
Treatment groups (n)
Results
Secondary prevention
Antiarrhythmics versus implantable defibrillators (AVID) (1997) [5]
Patients with VF arrest, sustained VT
Mean follow-up: 8.2 ± 12.2 months
Empiric amiodarone (356), randomized to amiodarone (79), amiodarone after sotalol failure (58), EPS-guided sotalol (13) vs. ICD implantation (507)
27 % mortality reduction after 2 years in ICD group
12.4 % of ICD group were later started on sotalol or amiodarone
9.8 % of antiarrhythmics group later received an ICD
Canadian Implantable Defibrillator Study (CIDS) (2000) [6]
Patients with VF arrest or sustained VT without recent MI or electrolyte abnormality
Mean follow-up: 2.9–3.0 years
Empiric amiodarone (331) vs. ICD implantation (328)
Cumulative 20 % risk reduction in mortality and 33 % reduction in arrhythmic mortality; 21.4 % of patients randomized to amiodarone received an ICD; 28.1 % of patients randomized to ICD were later placed on amiodarone
Cardiac Arrest Study Hamburg (CASH) (2000) [7]
Patients with VT/VF arrest not related to MI, cardiac surgery, electrolyte abnormalities, or proarrhythmia
Mean follow-up: 57 ± 34 months
Empiric amiodarone (92) vs. metoprolol (97) vs. ICD implantation (99)
The 58 patients randomized to propafenone were excluded later due to an interim analysis showing 61 % higher relative risk of all-cause mortality compared to ICD
Death rates were 36.4 % in ICD arm and 46.6 % in the combined metoprolol and amiodarone groups, but did not reach significance (p = 0.08) (no significant difference between amiodarone and metoprolol groups)
Primary prevention
Multicenter Unsustained Tachycardia Trial (MUSTT) (1993) [4]
Patients with ischemic cardiomyopathy and an LVEF ≤40 % with ≥3 beats asymptomatic unsustained VT ≥4 days after MI, without current exercise-induced ischemia; inducible sustained VT on EPS
Mean follow-up: 39 months
2,202 enrolled and underwent EPS; 767 had inducible sustained VT; 704 randomized; 351 received EPS-guided therapy, with 158 receiving antiarrhythmics and 161 receiving ICDs
2-year death rate in patients with no therapy 28 % vs 22 % in the patients treated with EPS-guided therapy; there was a 9 % risk of cardiac death in patients with an ICD compared to 37 % in patients without an ICD
Multicenter Automatic Defibrillator Implantation Trial (MADIT) (1996) [1]
LVEF ≤35 %; history of MI ≥3 weeks prior; revascularization ≥3 months prior; NYHA I–III; ≥3 beats asymptomatic unsustained VT; inducible sustained VT on EPS
Mean follow-up: 27 months
101 received conventional therapy; 95 received ICDs (45 transthoracic; 50 transvenous)
Stopped early because of significant reduction of mortality in ICD group (HR 0.46, CI 0.26–0.82); 74 % in conventional group on amiodarone; 11 in the conventional therapy group received an ICD
Prophylactic ICD after Coronary Artery Bypass Graft (CABG-Patch) (1997) [11]
LVEF ≤35 %, abnormal signal-averaged ECG
Mean follow-up: 32 months
Of 1422 eligible patients, 900 randomized to ICD (446) or control (454)
No significant difference in all-cause mortality or cardiac death between groups (HR 1.03; CI 0.75–1.41)
Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) (2002) [2]
LVEF ≤30 %, history of MI; revascularization ≥3 months prior; NYHA I–III
1232 patients randomized to defibrillator (742) or conventional therapy (490)
Stopped early because of significant reduction of mortality in ICD group (HR 0.69, CI 0.51–0.93); 22 patients in conventional therapy group received an ICD
Mean follow-up: 20 months
Cardiomyopathy Trial (CAT) (2002) [3]
Dilated cardiomyopathy within 9 months of diagnosis; LVEF ≤30 %, NYHA II–III
104 patients randomized to ICD (50) or control (54)
Underpowered, because mortality for all patients (5.6 %) was significantly below predicted; no significant difference between groups
Mean follow-up: 5.5 years
Amiodarone versus implantable cardioverter-defibrillator: randomized trial (AMIOVIRT) (2003) [4]
Nonischemic dilated cardiomyopathy with LVEF ≤35 %, asymptomatic nonsustained VT, NYHA classes I–III
103 patients randomized to ICD (51) or amiodarone (52) (mean dose 300 mg)
3-year survival (88 % in ICD group vs. 87 % with amiodarone) and arrhythmia-free survival (63 % in ICD group and 73 % with amiodarone) were not significantly different
Mean follow-up: 2 years
Defibrillator in Acute Myocardial Infarction Trial (DINAMIT) (2004) [10]
LVEF ≤35 % with an MI 6–40 days prior and reduced heart rate variability or elevated resting heart rate >80 beats/min; excluded patients with NYHA IV HF, recent CABG or 3-vessel PCI
674 patients randomized to ICD (332) or control (342); 36 % had PCI and 24 % had thrombolysis only; 95 % received ACE inhibitors and 87 % received beta-blockers
No difference in mortality was observed (HR 1.08; p = 0.66); the decrease in arrhythmic death in the ICD group was offset by an increased rate of death from nonarrhythmic causes
Mean follow-up: 30 months
Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) (2004) [5]
Nonischemic cardiomyopathy with LVEF ≤35 % with PVCs or nonsustained VT; NYHA classes I–III
458 patients randomized to standard medical therapy (229) or standard medical therapy with an ICD (229)
ICD significantly reduced mortality from arrhythmia (HR 0.20, CI 0.06–0.71), but there was no significant difference in all-cause mortality
Mean follow-up: 29 months
Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) [6]
Ischemic or nonischemic cardiomyopathy, EF ≤35 %, QRS ≥120 ms, NYHA classes III–IV; hospitalization for HF in the preceding year
Randomized to optimal medical therapy alone (308), optimal medical therapy with CRT-D (595), or optimal medical therapy with CRT-P (617)
26 % of patients within the control group withdrew to receive CRT devices; mortality was significantly higher in the control group (25 %), but not significantly different between the CRT-P (21.2 %) and the CRT-D (17.6 %) groups, with the greatest effect in the CRT-D group
Mean follow-up: 14.8 months in control group; 16 months in CRT-P and CRT-D groups
Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) (2005) [7]
LVEF ≤35 % from ischemic or nonischemic cause; NYHA classes II–III
2,521 patients randomized to placebo (847), amiodarone (median dose 300 mg) (845), and ICD (829)
ICD significantly reduced mortality (HR 0.77; CI 0.62–0.96); amiodarone did not significantly reduce mortality compared to placebo (HR 1.06); 11 % of medically treated patients received an ICD
Mean follow-up: 45.5 months
Certain types of VT in patients with structurally normal hearts respond well to medical therapy and catheter ablation; therefore, ICD implantation is not indicated in right ventricular outflow tract tachycardia, fascicular tachycardia, or idiopathic ventricular tachycardia originating from the aortic cusps. These tachycardias in a structurally normal heart have a low risk of causing sudden cardiac death.
52.1.2 Acute Approach to the Patient with a Ventricular Arrhythmia
The acute assessment of the patient requires a rapid assessment of hemodynamic stability as well as an evaluation for contributing conditions. The prerequisite to appropriate treatment is an understanding of the substrate responsible for the ventricular arrhythmias. This includes careful analysis of the ECG in sinus rhythm, the ECG in tachycardia if available, other laboratory investigations, echocardiogram, chest X-ray, and other indicated tests. Cardiac magnetic resonance imaging has become very useful for evaluating particular arrhythmogenic substrates, including myocarditis, cardiac sarcoidosis, arrhythmogenic right ventricular dysplasia/cardiomyopathy, hypertrophic cardiomyopathy, and ischemic cardiomyopathy.
52.1.3 Role of Antiarrhythmic Medications in the Acute and Chronic Treatment of Ventricular Arrhythmias
The acute treatment of ventricular arrhythmias often cannot be delayed for multiple investigations. In numerous studies, empiric therapy is non-inferior to electrophysiologic study-guided therapy and centers around two pharmacologic targets. First, a reduction in catecholamine stimulation to the heart is essential to suppress automaticity as well as triggered activity producing VT. Catecholamines are blocked with beta-adrenergic receptor antagonists (beta-blockers), and their production is suppressed by anxiolytics, sedation, general anesthesia, and sympathetic denervation in the form of a left stellate ganglion blockade or excision. Ion channel modulation by antiarrhythmic medication is chosen primarily on the basis of medications that are least likely to cause hemodynamic deterioration. As will be discussed below, since amiodarone is the most effective medication for ventricular arrhythmias over a wide variety of substrates, has less proarrhythmic potential than other antiarrhythmics, and has a relatively straightforward dosing regimen with no requirement for serum level monitoring in the acute setting, it is usually the antiarrhythmic of choice. The potential difficulties with amiodarone come after the acute arrhythmia is treated: the activity on ion channels may interfere with the inducibility of the arrhythmia in the electrophysiology laboratory; the prolonged half-life of this medication makes its effects long lasting; and the long-term toxicities to other organ systems all should be considered.
Despite a great number of innovations in the nonpharmacologic therapy of ventricular arrhythmias including catheter ablations and ICD therapies, antiarrhythmic drugs remain a cornerstone of therapy. Antiarrhythmic medications are often used in conjunction with ICD and catheter ablation to reduce the risk of tachycardia reoccurring. While acute pharmacologic cardioversion and maintenance of sinus rhythm in patients with an ICD are their primary uses, antiarrhythmic medications can be used in the electrophysiology lab to observe its effects during electrophysiology study and ablation. Therefore, in the management of patients with ventricular arrhythmias, antiarrhythmic medications are indispensable. This chapter will thoroughly review the potential uses of antiarrhythmic medications, particularly in the context of ventricular arrhythmias. Then, the pharmacology and clinical uses of each individual medication will be discussed.
52.2 Clinical Use of Antiarrhythmic Medications
52.2.1 Acute Pharmacologic Cardioversion
Pharmacologic cardioversion is an attractive alternative to direct-current cardioversion in conscious patients since it does not require sedation or cause discomfort. For successful pharmacologic cardioversion, antiarrhythmics must be administered in an intravenous formulation and must act rapidly. One of four medications can be used.
Procainamide is a sodium and potassium channel blocker but also functions as a negative inotrope. It can cause significant hypotension, particularly in patients with reduced systolic function. Therefore, it is useful only in hemodynamically stable patients with preserved ejection fraction. It is safely used in patients with preexcitation. Because supraventricular arrhythmias with preexcitation may be indistinguishable from VT originating from the base of the heart, procainamide can be used in this setting.
Lidocaine is a sodium channel blocker that exercises its antiarrhythmic effect on fast sodium channels; it most significantly affects ventricular tissues. Lidocaine preferentially binds to inactive sodium channels and thus has a more pronounced effect during acute ischemia. For this reason, lidocaine has historically been preferred for use in VT during acute ischemia, although amiodarone is also effective for this indication.
In patients presenting with VT, whether monomorphic stable VT or VT/VF arrest, amiodarone has been shown repeatedly to be superior to lidocaine [12–14]. While oral amiodarone takes time to load to reach effective levels, intravenous amiodarone is effective acutely and can be loaded safely [15, 16, 20]. Some formulations have diluents (polysorbate 80 and benzyl alcohol) that can cause hypotension; other formulations do not.
The potassium channel blocker nifekalant (available in Japan) has also been studied in the acute setting to treat malignant ventricular arrhythmias, including arrhythmias during acute ischemia [17, 18]. As a potassium channel blocker, it does have the potential to cause torsade de pointes by prolonging the QT interval.
The treatment of pulseless VT or ventricular fibrillation (VF) cardiac arrest also involves using antiarrhythmic medications in addition to pressors if the patient cannot be successfully defibrillated. Amiodarone, lidocaine, bretylium, and nifekalant have been compared in randomized trials. Amiodarone is more effective than lidocaine, with 12 % of patients treated with lidocaine surviving to hospital admission compared to 23 % with amiodarone [19]. Nifekalant is also more effective than lidocaine in VT/VF arrest [19, 20]. Bretylium prevents the release of norepinephrine and also blocks potassium channels. Although it is as effective as amiodarone in converting ventricular arrhythmias to sinus rhythm, bretylium causes severe hypotension because of the effects on norepinephrine release, and it is no longer available in the USA [21]. Neither nifekalant nor amiodarone reduces the systolic function; and while both block potassium channels, torsade de pointes is rare [22, 24]. In the one randomized study of nifekalant and amiodarone in VT/VF arrest, amiodarone appeared to be more effective in converting patients to sinus rhythm [23].
52.2.2 Preventing Recurrence of Ventricular Arrhythmias
In patients with structural heart disease and sustained ventricular arrhythmias or sudden cardiac death, implantable cardioverter-defibrillators (ICDs) reduce mortality, with a 28 % relative risk reduction in mortality and a 50 % reduction in arrhythmic death [8]. Although the ICD treats ventricular arrhythmias, it does not prevent their recurrence. ICD shocks are associated with significant discomfort and distress and can cause anxiety disorders, including posttraumatic stress disorder. Antiarrhythmics can prevent ventricular arrhythmia recurrence.
In choosing which antiarrhythmic to use to prevent VT or VF or to suppress PVCs, many factors must be taken into account. The patient’s substrate, comorbid conditions, age, and frequency of ventricular arrhythmias must be taken into account. Initial comparison studies demonstrated that electrophysiologic study was not an effective method of guiding medical therapy [24]. Later, the combination of beta-blockers with amiodarone was demonstrated to suppress ventricular arrhythmias more effectively than sotalol or beta-blockers alone, with a hazard ratio of 0.27. However, the side effects and toxicities associated with amiodarone led to a discontinuation rate of 18 % at 1 year [25]. Because of these toxicities, it is reasonable to delay initiating amiodarone until after other therapies have failed. On the other hand, patients with frequent VT and multiple shocks should likely be treated with the most effective regimen, beta-blocker with amiodarone.
The multitude of antiarrhythmic choices can be confusing. However, the vast majority of patients with ventricular tachycardia presenting to a hospital will have a history of myocardial infarction, heart failure, or renal dysfunction, all of which significantly limit the available options. Essentially, the only antiarrhythmics found to be safe in patients with heart failure are amiodarone, dofetilide, and lidocaine or mexiletine. If the patient is having acute coronary syndrome, then lidocaine or amiodarone can be used. Sotalol could be considered for chronic treatment in patients with ischemic cardiomyopathy, but not with an ejection fraction (EF) <40 %. Significant renal insufficiency limits the choice of antiarrhythmics to amiodarone, mexiletine, or propafenone. Table 52.2 lists the medications (with standard doses) that can be useful for the treatment of VT.
Table 52.2
Drugs and doses for treating ventricular arrhythmias
Drug | Indication | Initiation | Maintenance | Dose reduction |
|---|---|---|---|---|
Quinidine | Chronic treatment (≤600 mg/day for prevention of VF in Brugada syndrome) | Quinidine sulfate immediate release 200–400 mg q6h | Quinidine sulfate: 300 mg q8h–q12h | Reduce by 25 % if CrCl <10 mL/min |
Quinidine gluconate: 324 mg q8h–q12h | ||||
Procainamide | Acute treatment | 17 mg/kg at 20–50 mg/min | 1–4 mg/min | Reduce infusion by 33 % for CrCl 10–50 mL/min; reduce by 67 % for CrCl <10 mL/min |
Procainamide challenge to uncover Brugada ECG | 10 mg/kg at 50 mg/min | Not applicable | ||
Lidocaine | VT/VF arrest or monomorphic VT | 1 mg/kg first bolus; 0.5–0.75 mg/kg subsequent boluses | 1–3 mg/min | Hepatic failure or heart failure |
Plasma levels: 1.5–5.0 mcg/mL | ||||
Mexiletine | Chronic treatment | 150 mg q8h | 150–300 mg q8h | Hepatic failure or heart failure |
Flecainide | Chronic treatment | 100 mg q12h | 100 mg q12h; max 200 mg q12h | Reduce by 50 % if CrCl <50 mL/min |
Esmolol | Acute beta blockade | 0.5 mg/kg bolus | 0.05 mg/kg/min infusion | Titrate to effect |
Amiodarone | VT/VF arrest | 300 mg bolus | 400–800 mg q12h for 1–2 weeks, then 400–800 mg daily; reduce dose to 400 mg daily if possible | Hepatic enzymes >3× upper limit of normal |
Stable VT | 150 mg bolus, then 1 mg/min × 6 h, then 0.5 mg/min × 12 h; repeat 150 mg bolus if VT recurs | |||
Nifekalant | VT/VF arrest | 0.2–0.3 mg/kg bolus | 0.2 mg/kg/h drip | Reduce 50 % for CrCl <50 mL/min |
Sotalol | Chronic treatment | 80 mg q12h; increase dose every 3 days | 240 mg/day divided into 2–3 doses | Dose q24h for CrCl 30–60 mL/min; dose q36h–q48h for CrCl 10–30 mL/min |
Acutely, patients with electrical storm who have been urgently cardioverted, whether externally or internally by their ICDs, require medication to prevent further ventricular arrhythmias and shocks. For most patients, this requires normalizing electrolytes, suppressing catecholamines with beta-adrenergic antagonists, modulating cardiac ion channels with antiarrhythmics such as amiodarone and lidocaine, and suppressing the sympathetic drive with sedation, analgesia, or even general anesthesia. In patients with heart failure, hemodynamic support can reduce the occurrence of ventricular arrhythmias. Ultimately, catheter ablation may provide definitive nonpharmacologic therapy to prevent recurrence of ventricular arrhythmias. Figure 52.1 illustrates an algorithmic approach to suppressing ventricular arrhythmias in electrical storm.
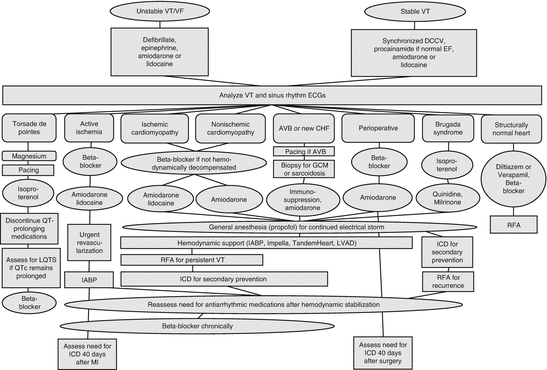

Fig. 52.1
An algorithmic approach to suppressing ventricular arrhythmias and stabilizing patients in electrical storm. Abbreviations: AVB atrioventricular block, CHF congestive heart failure, DCCV direct-current cardioversion, ECG electrocardiogram, EF ejection fraction, GCM giant cell myocarditis, IABP intra-aortic balloon pump, ICD implantable cardioverter-defibrillator, LQTS long QT syndrome, LVAD left ventricular assist device, MI myocardial infarction, QTc corrected QT interval, RFA radiofrequency ablation, VF ventricular fibrillation, VT ventricular tachycardia
52.2.3 Neurohormonal Modulation to Reduce Mortality
Beta-adrenergic antagonists, angiotensin-converting enzyme inhibitors, angiotensin-II AT1 receptor blockers, and aldosterone receptor antagonists have been proven to reduce all-cause and cardiovascular mortality in patients with reduced ejection fraction or patients with myocardial infarction in multiple trials. Meta-analyses indicate substantial reduction in arrhythmic mortality as well. Beta-blockers reduce the relative risk of sudden cardiac death in patients with heart failure by 31 %, with the vast majority of the data in patients on metoprolol or carvedilol [26]. After myocardial infarction, angiotensin-converting enzyme inhibitors reduce the relative risk of sudden cardiac death by 20 % [27]. The aldosterone antagonists (spironolactone and eplerenone) reduce the odds of sudden cardiac death by 23 % in patients with an ejection fraction ≤45 % [28]. These medications are more extensively discussed in prior chapters on medical management of heart failure, ischemic coronary artery disease, and hypertension, but they deserve mention here as essential components in managing patients with ventricular arrhythmias with these substrates (Chaps. 5, 8, 20, 36 and 38).
52.2.4 Effect on Defibrillation Threshold
By affecting the membrane potential, amiodarone raises the defibrillation threshold, and sotalol decreases the defibrillation threshold [29]. This is rarely clinically significant, with changes averaging less than 2 J. Reassessment of defibrillation threshold after changing medication is not recommended.
52.2.5 Use of Antiarrhythmics in the Electrophysiology Lab
By using procainamide as a continuous infusion in the electrophysiology lab to slow a monomorphic VT, the VT cycle length slows as fast sodium channels are blocked. A slower VT cycle length is more likely to be hemodynamically stable, so patients can tolerate being in VT during mapping and ablation. Additionally, procainamide, ajmaline (not available in the USA), or flecainide can be used in patients in whom Brugada syndrome is suspected to uncover a type 1 Brugada ECG pattern in order to determine the patient’s risk of future ventricular arrhythmias [30].
52.3 Specific Uses for Antiarrhythmic Drugs
52.3.1 Classification
The most widely known classification of antiarrhythmic drugs is the Vaughan-Williams classification, proposed in the 1970s. The classification is based on the preeminent property of the particular antiarrhythmic, despite the fact that many antiarrhythmics have effects on more than one ion channel or receptor.
Four classes were proposed: class I, which contains drugs that block the inward sodium current (I Na), is subdivided into three subgroups. Group 1a slows conduction by blocking sodium channels but also prolongs repolarization by blocking potassium channels. Group 1b antiarrhythmics have minimal effect on the rate of conduction, because they bind to the sodium channel in the inactive state, and shorten repolarization. Group 1c slows conduction by inactivating active sodium channels and has no effect on repolarization. Class II contains most of the beta-adrenergic receptor blockers; however, sotalol, a nonselective beta blocker, is classified with class III because it also blocks potassium channels. All antiarrhythmics in class III prolong repolarization because they block potassium channels. Finally, class IV includes only the L-type calcium channel blockers that inhibit the inward calcium current (I Ca-L), the non-dihydropyridine calcium channel blockers diltiazem and verapamil (Chap. 37). Drugs such as digoxin, ivabradine, and adenosine that are also used to treat arrhythmias are not included in this classification. Since ibutilide, digoxin, and ivabradine have no role in treating ventricular arrhythmias, they will not be discussed further in this chapter. The primary ion currents affecting the cardiac action potential and the drugs modulating those currents are depicted in Fig. 52.2 (also see Chap. 46).
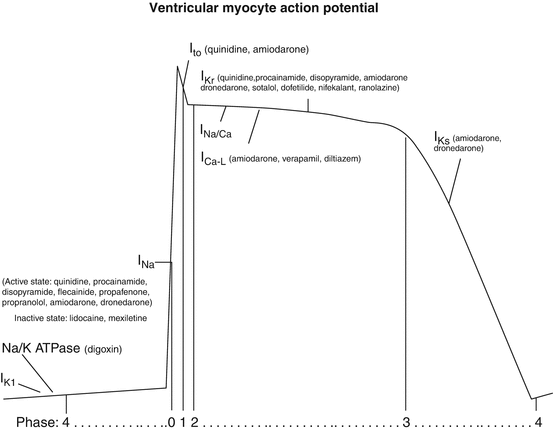

Fig. 52.2
The ventricular myocyte action potential, contributing ionic currents, and the primary pharmacologic agents that affect those currents. Abbreviations: I Ca-L L-type calcium channel current, I K1 inward rectifier potassium channel, I Kr rapid delayed rectifier potassium current, I Ks slow delayed rectifier potassium current, I Na fast inward sodium current, I to transient outward potassium current, I K1 inward rectifier potassium channel, I Kr rapid delayed rectifier potassium current, I Ks slow delayed rectifier potassium current, I Kur ultra-rapid delayed rectifier potassium current, I Na fast inward sodium current, I Na/Ca sodium/calcium exchanger, I to transient outward potassium current, Na/K ATPase sodium/potassium adenosine triphosphatase
Following further investigations, the limitations of the Vaughan-Williams classification became increasingly apparent. The European Society of Cardiology Working Group on Arrhythmias attempted in 1991 to reclassify the antiarrhythmics based on electrophysiologic effects in a scheme called the Sicilian Gambit [31]. The goal was to classify antiarrhythmics based on the predominant clinically important effect, rather than the in vitro targets. It focuses on the impact each antiarrhythmic has on specific vulnerable parameters related to the arrhythmia mechanism. While this classification is advantageous in that it is flexible to incorporate many additional medications with various mechanisms of action, it has not obtained widespread utilization due to its complexity; therefore, the Vaughan-Williams classification will provide the organizational structure for further discussion of antiarrhythmics. The pharmacologic and pharmacokinetic properties of each antiarrhythmic drug are compared in Table 52.3. A table based on the Sicilian Gambit reflects the action of each antiarrhythmic as shown in Table 52.4.
Table 52.3
Major electrophysiologic actions, pharmacokinetics, adverse effects, and indications of antiarrhythmics commonly used to treat ventricular tachyarrhythmias
Class | Antiarrhythmic | Mechanism of action (channels inhibited) | Electrophysiologic effects | Pharmacokinetics | Noncardiac adverse effects | Cardiac adverse effects | Indications |
|---|---|---|---|---|---|---|---|
IA | Quinidine (quinidine sulfate has 75 % the quinidine base as does quinidine gluconate) | I Na (intermediate recovery, moderate blockade), I to, I Kr, muscarinic, α | QRS prolonged | Vd 2–3 L/kg, less in CHF | Lightheaded, diarrhea, nausea, emesis, tinnitus, blurred vision, rash, weakness, tremor, blood dyscrasias | QRS and QTc prolonged; AVN block; TdP; syncope; toxicity worse with digoxin | AF, VT, BrS, SQTS, VF with early repolarization |
JT prolonged | |||||||
b: 70 % | |||||||
t 1/2:6–8 h longer in CHF | |||||||
Metab: H | |||||||
Excr: U | |||||||
Preg: C | |||||||
APD prolonged; HV, AERP, and VERP prolonged | |||||||
IA | Procainamide | I Na (intermediate recovery, moderate blockade), I Kr | QRS prolonged | Vd 2 L/kg, less in CHF; Metab: H | Lupus symptoms, diarrhea, nausea, blood dyscrasias | QTc and QRS prolonged; AVN block | AF, VT, WPW, unmasking BrS |
JT prolonged | |||||||
APD prolonged; HV, AERP, and VERP prolonged | t 1/2: 2–5 h; NAPA 6–8 h | ||||||
Excr: U (50 % procainamide, 80 % NAPA) | |||||||
Preg: C | |||||||
IA | Disopyramide | I Na (intermediate recovery, moderate blockade), I to, I Kr, I K(ATP), muscarinic | QRS prolonged | Vd: 0.8–2 L/kg | Xerostomia, constipation, urinary hesitancy, rash | Hypotension, CHF, syncope, QTc prolonged | AF, VT, HCM |
JT prolonged | b : 60–83 % | ||||||
t 1/2 : 4–10 h | |||||||
APD prolonged; HV, AERP, and VERP prolonged | Metab : H | ||||||
Excr : U 80 % | |||||||
Preg: C | |||||||
IB | Lidocaine | I Na (rapid recovery, mild blockade) | No marked effect on most intervals; APD and JT can slightly shorten | Vd: 1.1–2.1 L/kg, less in CHF | Delirium, psychosis, seizure, nausea, tinnitus, dyspnea, bronchospasm | Bradycardia, hemodynamic collapse, AVN block | VT, VF |
Biphasic t 1/2: 7–30 m, 90–120 m | |||||||
Metab: H | |||||||
Excr: U | |||||||
Preg: B | |||||||
IB | Mexiletine | I Na (rapid recovery, mild blockade) | No marked effect on most intervals; APD and JT can slightly shorten | Vd: 5–7 L/kg | Lightheaded, tremor, ataxia, paresthesias, blood dyscrasias | CHF, AVN block | VT, LQTS 3 |
b: 50–60 % | |||||||
t 1/2: 10–14 h | |||||||
Metab: H | |||||||
Excr: U | |||||||
Preg: C | |||||||
IC | Flecainide | I Na (slow recovery, marked blockade), I Kr, I Kur | PR prolonged | Vd: 5–13 L/kg | Dizziness, tremor, vision disturbance, dyspnea, nausea | Sinus node dysfunction, AVN block, prolonged QRS | AF, AVNRT, VT, CPVT |
QRS prolonged | b: 85–90 % | ||||||
APD prolonged | t 1/2: 7–22 h | ||||||
AH, HV prolonged | Metab: H | ||||||
Excr: U | |||||||
Preg: C | |||||||
IC | Propafenone | I Na (slow recovery, marked blockade), I Kr, I Kur, β, α | PR prolonged | Vd: 252 L | Dizziness, fatigue, nausea, diarrhea, zerostomia, tremor, blurred vision | AVN block, VT, CHF, QRS prolonged | AF, VT |
QRS prolonged | b: 3–10 % | ||||||
APD prolonged | t 1/2: 2–10 h or 10–32 h | ||||||
AH, HV, and VERP prolonged | |||||||
Metab: H | |||||||
Excr: U | |||||||
Preg: C | |||||||
II | Atenolol | β1 | Sinus rate slowed | b: 50 % | Dizziness, fatigue, depression, impotence | Bradycardia, hypotension, | AF, AFL, AT, AVNRT, VT, CPVT, |
t 1/2: 6–7 h | |||||||
ERP–AVN prolonged | Excr: F 50 %, U 40 % | CHF, AV block | LQTS, OTVT | ||||
Preg: D | |||||||
II | Carvedilol | β1, β2, α | Sinus rate slowed | Vd: 115 L | Hyperglycemia, dizziness, fatigue, diarrhea | Bradycardia, hypotension, AV block, syncope, edema | AF, AFL, AT, VT, HCM, ICM, NICM |
b: 25–35 % | |||||||
ERP–AVN prolonged | |||||||
t 1/2: 7–10 h | |||||||
Metab: H | |||||||
Excr: F | |||||||
Preg: C | |||||||
II | Esmolol | β1 < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|