Limitations
Drugs are widely used in the treatment of arrhythmias, but their limitations should be appreciated. Antiarrhythmic drugs are of limited effectiveness: in other words, a drug prescribed in the correct dose for an appropriate indication may fail to work. With many drugs it is difficult to maintain consistent therapeutic drug levels. Considerable insight into the mode of action of antiarrhythmic drugs has been gained, but selection for an individual patient of a drug that is both effective and well tolerated is often a process of trial and error. Unwanted effects often occur: the most common are hypotension, heart failure, impairment of the specialised cardiac conducting tissues and symptoms from the gastrointestinal and central nervous systems.
Proarrhythmic effect
Many antiarrhythmic drugs, particularly those in class IA and IC (see below), can sometimes worsen or cause arrhythmias, sometimes with fatal consequences. Patients with poor ventricular function are at greatest risk, while the risk is low in those with structurally normal hearts.
Choice of treatment
Drugs are only one form of treatment, and in some situations other approaches – i.e. catheter ablation, cardioversion, artificial pacing or surgery – may be better.
A number of factors influence the choice of treatment: the type of arrhythmia, the urgency of the situation, the need for short- or long-term therapy and the presence of impaired myocardial performance, sick sinus syndrome or abnormal AV conduction.
It is important to consider whether an antiarrhythmic drug is being given to terminate an arrhythmia, to prevent its recurrence or to slow the heart rate during the arrhythmia. In some situations drugs are given to control symptoms, whereas in others the purpose may be to prevent dangerous arrhythmias.
Modes of action
The modes of action of antiarrhythmic drugs can be classified according to their effects in the intact heart (clinical classification) or according to their effects at cellular level as established by in vitro studies (action potential classification). The latter classification is widely referred to, although it is of limited practical value.
Clinical classification
In this classification, drugs are divided into three groups according to their main site or sites of action in the intact heart.
| Classification of antiarrhythmic drugs according to principal site(s) of action in intact heart | |
| Site of action | Examples |
| AV node | Verapamil, diltiazem, adenosine, digoxin, beta-blockers |
| Ventricles | Lignocaine, mexiletine |
| Atria, ventricles, accessory AV pathways | Quinidine, disopyramide, amiodarone, flecainide, procainamide, sotalol, propafenone |
The first group consists of drugs whose chief action is to slow conduction in the atrioventricular (AV) node. These drugs may therefore be useful in the treatment of arrhythmias of supraventricular origin. In the second group are drugs that work mainly in ventricular arrhythmias. The third group comprises drugs that act on the atria, ventricles and, in cases of Wolff–Parkinson–White syndrome or AV re-entrant tachycardia, accessory AV pathways. Thus, they may be effective in both supraventricular and ventricular arrhythmias.
Action potential classification
In this classification, drugs are divided into four main classes depending upon their electrophysiological effects at cellular level.
Class I
Class I drugs impede the transport of sodium across the cell membrane during the initiation of cellular activation and thereby reduce the rate of rise of the action potential (phase 0). They are subdivided into classes A, B and C according to their effect on the duration of the action potential (which is reflected in the surface ECG by the QT interval). IA drugs increase the duration of the action potential, IB drugs shorten it and IC drugs have little effect. The antiarrhythmic action of IB drugs is confined to the ventricles, whereas IA and IC drugs affect both atria and ventricles. IA and particularly IC drugs slow intraventricular conduction, and IA drugs can significantly prolong the QT interval.
Class II drugs
Class II drugs interfere with the effects of the sympathetic nervous system on the heart. They do not affect the action potential of most myocardial cells but do reduce the slope of spontaneous depolarisation (phase 4) of cells with pacemaker activity and thus the rate of pacemaker discharge.
Class III drugs
Class III drugs block potassium ion transport across the cell membrane. They prolong the duration of the action potential and hence the length of the refractory period and the QT interval, but do not slow phase 0.
Class IV drugs
Class IV drugs antagonise the transport of calcium across the cell membrane which follows the inward flux of sodium during cellular activation. Cells in the AV and sinus nodes are particularly susceptible. It should be noted that the dihydropyridine calcium channel blockers, nifedipine and amlodipine, do not have an antiarrhythmic action.
Limitations of action potential classification
The majority of drugs are in class I, and drugs within this class differ significantly in their clinical effects. Some drugs have more than one class of action: amiodarone has class I, II and IV actions as well as its main class III effect! Furthermore, some drugs (e.g. digoxin and adenosine) cannot be classified.
Notes on individual drugs
Flecainide
Flecainide is a potent drug which can be given both orally and intravenously. Its indications include paroxysmal atrial fibrillation, pre-excitation syndromes and ventricular arrhythmias. It is very effective at suppressing ventricular ectopic beats but less so in the treatment of ventricular tachycardia.
It has a long half-life of approximately 16 hours, which facilitates twice-daily oral administration. The usual oral dosage is 100 mg twice daily. If side effects occur then reduction of the daily dosage by as little as 50 mg can help. Rarely, a twice-daily dose of 50 mg is effective, and occasionally 300 mg daily is required. There is a once-daily, slow-release preparation available, the dose being 200 mg daily.
The intravenous dose is 1–2 mg/kg body weight over 10 minutes; it should be given more slowly in patients with impaired ventricular function. Flecainide is both metabolised by the liver and excreted by the kidney.
The drug has a narrow therapeutic range, i.e. it can be difficult to achieve a therapeutic action without unwanted effects. High levels can cause visual disturbance, particularly on rotating the head, light-headedness and nausea. Marked QRS prolongation (> 25%) during treatment indicates that the blood level may be too high. The drug has been shown to increase the threshold of pacemaker stimuli.
The drug does have an important negative inotropic action and should be avoided in patients in heart failure or with extensive myocardial damage. It can be proarrhythmic, particularly in patients with a history of sustained ventricular tachycardia and/or poor ventricular function. In a major study of patients with ventricular extrasystoles following myocardial infarction, flecainide was found to increase mortality. It is now generally agreed that the drug should not be given to patients known to have coronary artery disease.
Flecainide causes slight prolongation of the QRS complex and hence the QT interval: it does not prolong the JT component of the QT interval, as do quinidine and disopyramide.
Flecainide is effective and safe when used to prevent atrial fibrillation and AV re-entrant tachycardias in patients with structurally normal hearts. It is also indicated in patients with highly symptomatic idiopathic ventricular extrasystoles.
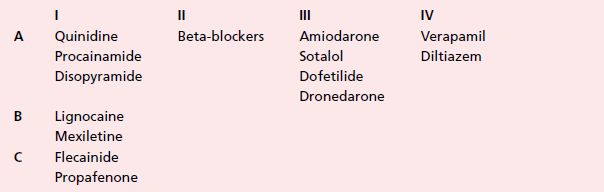
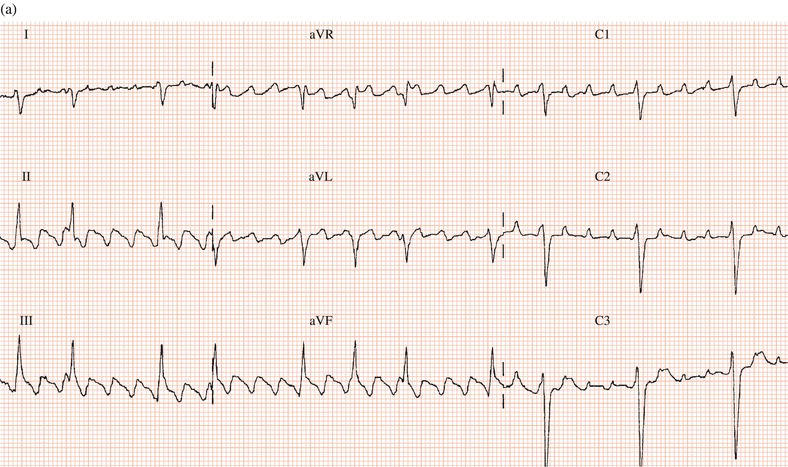
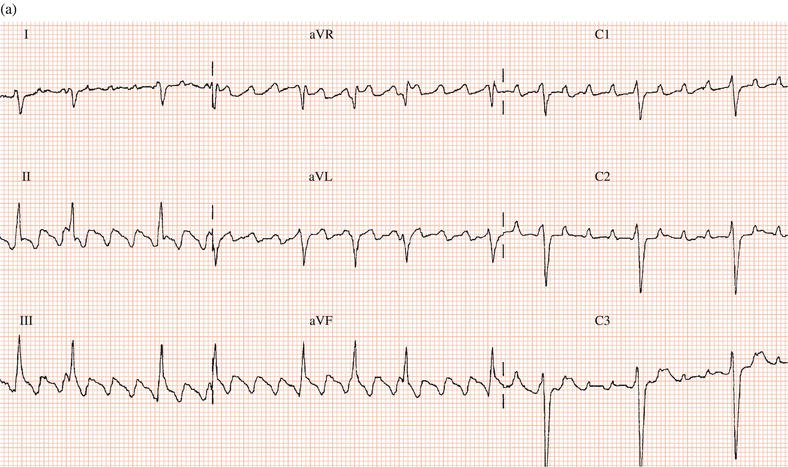
Occasionally, like other class I drugs, flecainide can worsen atrial arrhythmias: either converting atrial fibrillation to flutter or increasing the ventricular rate during atrial flutter (Figure 19.1).
The drug must not be given to patients with the Brugada syndrome.
Propafenone
This drug has both IC and mild beta-blocking properties and has been shown to be effective in both supraventricular and ventricular arrhythmias. It can be proarrhythmic and should not be given to patients with impaired ventricular function or with the Brugada syndrome. In the author’s experience, non-cardiac unwanted effects are common.
Amiodarone
Amiodarone has several advantages over other drugs. It is highly effective in both supraventricular and ventricular rhythm disorders: even in arrhythmias refractory to other drugs there is a 70% success rate. It has a remarkably long half-life (20–100 days), so that the drug need only be given once daily or even less frequently. It does not significantly impair ventricular performance and can be given to patients in heart failure. However, amiodarone has important unwanted effects, dictating that its long-term use is confined to patients with arrhythmias that are dangerous or resistant to other drugs, or where the risk of side effects is not a major consideration because the patient’s prognosis is poor, e.g. in the elderly and those with severe myocardial damage.
Though it is effective in the treatment of ventricular arrhythmias, recent studies have shown that it has no role in ‘primary prevention’ in patients with poor ventricular function, i.e. it does not reduce the incidence of fatal ventricular arrhythmias in these patients. Nevertheless, many patients with implantable defibrillators require amiodarone to reduce ventricular arrhythmias in order to prevent frequent shock delivery.
Figure 19.1 (a) Patient with atrial flutter with 2:1 AV conduction. (b) Same patient after flecainide: the drug slowed the atrial rate, facilitating 1:1 AV conduction and consequently a marked increase in ventricular rate.
Unwanted effects
Short-term treatment with intravenous amiodarone is unlikely to cause side effects, although a few cases of hepatitis associated with the drug have been described. It often causes phlebitis if administered via a peripheral vein.
Longer-term oral therapy is associated with a high incidence of side effects.
Dermatological
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


