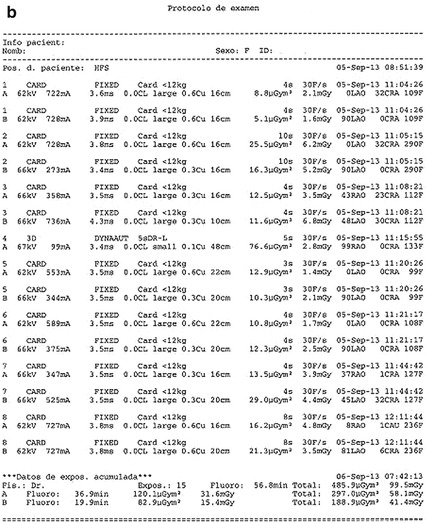
Fig. 5.1
(a, b) Dosimetric doses report in two regular angiographic studies. Total DPA dose related to cine acquisition with frame/s and projection. Radiation in the cath lab is generated using two different modes: fluoroscopy or “cine” angiography. Fluoroscopy involves 95 % of the total x-ray operation time but only causes 40 % of the total radiation dose; cine represents only 5 % of the total x-ray tube operation time but 60 % of the total radiation exposure
In computed tomography (CT), dose length product (DLP) is the standard dose measurement reported, expressed in mGy.cm. Important differences in doses are obtained with various CT protocols with saving algorithms, with/without cardiac gating, type of gating (prospective/retrospective), etc. Early studies with retrospective gating and no dose modulations delivered doses >15 mSv. Estimated lifetime risk of cancer is as high as 1:143 for a 20-year-old woman if a scan was performed retrospectively with no modulation (old explorations).
The scanner-derived DLP and the catheterization-derived DAP do not allow comparisons. Effective dose (mSv) allows cross-modality comparisons of radiation doses. In cardiac CT scan, the most common method of estimating the effective dose is the use of a conversion factor applied to the dose length product (DLP); conversion factors previously used in the literature varied between 0.014 and 0.019 mSv/mGy·cm and are based on tissue weightings published and updated by the ICRP. There are also PC-based programs that calculate effective doses with data from DAP, projection angle, kV, field size, duration of exposure, frames per second during acquisition, and patients’ height and weight. Conversion factors for conventional invasive coronary angiography have also been used but are based on older ICRP tissue weightings; published conversion factors vary widely from 0.12 to 0.26 mSv/Gy cm2 with 0.26 mSv/Gy cm2 the most common frequently simplified to 0.20 mSv/Gy × cm2. An average cardiac computed tomography and angiographic examination are equivalent to almost 100 and 300 chest x-rays, respectively (a single chest x-ray = 0.02 mSv).
5.5 How to Manage Radiation Doses for Invasive Cardiac Procedures [1]
5.5.1 Preprocedure
Use dosimeters and proper shielding: shields, lead curtains, aprons, and glasses. Learn and know about radioprotection and know your own screen dose assessment. Study on your equipment how to store fluoro, adjust pulse, and frame rate. Plan your study.
5.5.2 Procedure
Keep in mind the current mandate and use radiation “as low as reasonably achievable” (ALARA concept). Limit fluoro and cine. Store fluoro when image quality is not required. Achieving an adequate image, as opposed to the highest quality image that is possible, is a basic principle. Image intensifier low-level modes should be used as often as possible: try to work, both in fluoroscopy and in acquisition modes, with the lowest frame rate. Distance between the x-ray tube and the patient should be maximized, keeping the intensifier or flat-panel detector as close to the patient as possible. Use the lowest degree of magnification required for accurate interpretation. Minimize radiographic beam time (“cine” acquisition creates 12–20-fold higher-dose intensities than fluoroscopy mode). Acquisitions represent 60–70 % of the total DAP during a typical study. Collimation is an efficient radiation-reducing factor, and modern systems have virtual control of collimation. There are less irradiating angulations: 20° right anterior oblique gets the lowest patient DAP, cranial and caudal angulations rose the doses significantly and are maximum in left lateral angulations. The operator fatigue also raised radiation exposure to 28 % due to more and longer radiographic runs after working for more than 6 h. Remember, an adequate use of filters, especially for small (<15 kg) and the simpler rule than doubling the source to operator distance will decrease operator dose to approximately one quarter. Operator and personnel exposure are directly related to the dose area product: when operating in a biplane cine-acquisition mode, scattered radiation multiplies by a factor between 5 and 21. Pediatric cardiologists could easily achieve a lens opacification threshold so they should wear glasses, apron, and collars and should correctly use shields, curtains, etc. (Fig. 5.2).
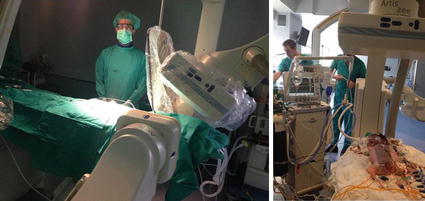

Fig. 5.2
Pediatric patients are often very sick and need ventilator, pumps, GE tubes, etc. Patient should be close to flat detector or intensifier; operator should use shields, curtains, glasses, collars, and apron. All these cautions make often impossible to achieve convenient angulations or biplane combinations to allow catheter, sheaths, or wire manipulations in small children
5.5.3 Postprocedure
Document radiation dose in records. Follow high-dose patients for the next weeks and refer to appropriate consultant if skin effects appear.
5.6 Angiographic Projections
Structural heart procedures are focused on chambers and structures larger than the 3-mm field of coronary procedures and are at greater risk of the limitations and artifacts of the Z-axis. Angiography produces a two-dimensional projection, and so multiple orthogonal views to minimize the impact of the “Z-axis” on the image are needed to understand heart and vessels in three dimensions. In the past, x-ray systems were fixed, and oblique and angled projections were achieved by changing the position of the patient on the table. Fortunately today, modern C-arms can be turned to get such projections.
The main idea is to get axial, non-overlapped, or foreshortened profile of the various structures; many and different angulations will be needed with great variations for the same structure or disease in different patients. Although bidimensional vascular angiogram will continue to lay a central role, advanced digital imaging and detailed three-dimensional reconstructions will enable more accurate diagnosis, minimizing contrast dye burden and radiation to patient and operators.
Every case should be prepared in anticipation, paying special attention to previous angiographies, CT, and MRI because in most cases, the projection which will optimize the profile could be anticipated looking at 3D reconstructions coming from these studies. Imaging workstations include packages that attempt to facilitate multimodality (fluoroscopy, ultrasound, CT, and MRI) fusion, but there are still important limitations for their use in real time such as the time consumed and the requirement of an expertise not easy to be gained; for instance, special attention needs to be paid to measurements as there are important differences for the same lesion between different techniques. Do not forget that angiographic images have a better temporal resolution, whereas MRI and CT images are merged pictures of the diameters during the whole cardiac cycle. The future will be the integration of imaging modalities; the information gained by one technique enhances and is incorporated in an additive manner to the information acquired by other technique.
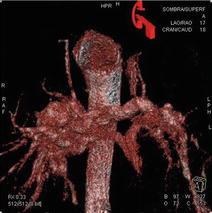
Angiographic computed tomography (ACT) provides cross-sectional CT images from a rotational angiography run using a C-arm-mounted flat-panel detector. The volume set obtained can be manipulated on a separate workstation in the interventional suite to generate a 3-dimensional angiographic picture combined with CT-quality soft tissue imaging that can be used in real time during the procedure. ACT is useful to define the optimal camera angles (Fig. 5.3) for a planned intervention under standard biplane or single-plane fluoroscopic guidance allowing to choose the best oblique angulations. There is still limited experience with this modality that needs some extra training: images obtained are time-averaged over the duration of 5-s arm rotation over 210ª, there are dropouts of the signal in areas of very tight stenosis or adjacent to stented regions, images are hand manipulated by the degree of windowing during post-processing, etc. Anyway, in selected cases, ACT has advantages over conventional CT: images are easily obtained in the same procedure and can be used to guide catheter manipulations serving as a 3D overlay road mapping, total radiation seems to be similar to length cine acquisition, and contrast dose is less or equal to that used to CT. But we lack enough information on the added benefits of that technique. Do we need the additional radiation and contrast exposure to perform preprocedural CT scans and C-arm procedural scans on some patients?
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


