Fig. 2.1
Theoretic cross sections of a coronary artery at different stages in the evolution to STEMI. The left panel demonstrates a TCFA with positive remodeling and only mild luminal narrowing. The second panel from the left depicts an artery with some progression and an asymptomatic plaque rupture with intraluminal thrombus formation. The lumen begins to narrow. This asymptomatic progression to STEMI is accelerated in the days to weeks prior to the event. The third panel depicts the thrombosed plaque of an acute STEMI that has completely obliterated the lumen with acute thrombus over layered thrombus. Following thrombolytic therapy or mechanical thrombectomy, the last panel depicts an open but still significantly narrowed arterial cross section with both plaque and residual thrombus occluding the lumen (Reproduced from Giampaolo et al. [43], with permission of Elsevier)
Angiographic Narrowing Remote from AMI
In addition to the early retrospective angiographic studies alluded to above indicating that about 70 % had <50 % diameter stenosis and only 13 % were >70 % stenosed, other more recent studies have supported this concept although in some cases, the diagnosis was not STEMI but other acute syndromes. These data are presented to indicate a pattern seen in many but not all studies related to the pathogenesis of ACS and AMI.
In 2005, Glaser et al. reported on 216 patients from the NHLBI Dynamic PCI Registry who required an additional angiogram for clinical progression at 1 year of a non target lesion [9]. Fifty nine percent presented with unstable angina and 9 % presented with non fatal MI. Of the 216, 157 were available for independent evaluation. The mean stenosis of the progressed lesion was 41.8 ± 20.8 % at the initial angiogram. A majority of lesions (60.5–95/157) were <50 % in severity at the time of the initial angiogram and only 13 % were >70 %. While studied at a different time and in a different population, these percentages were similar to the data reported in the 1980s.
Furthermore, in the PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) study, non culprit events (not the culprit lesion treated during the first angiogram) represented nearly 50 % of all repeat cardiovascular events at 3 years. While these new ischemic events included very few acute infarctions, IVUS in a sub group of these lesions with visual angiographic narrowing >30 % indicated that the responsible lesion at baseline was often a thin-capped fibroatheroma (TCFA) with a large plaque volume and a small cross sectional area. Nevertheless, the mean angiographic diameter stenosis at baseline was 32.3 ± 20.6 % and 59 % of lesions had a <50 % diameter stenosis. Thirty percent were <30 % stenotic at baseline and it is uncertain how many of these were interrogated with IVUS as only 52 % of non culprit lesions with events had accompanying IVUS data [10]. Thus, large plaques may not be significantly narrowed due to positive remodeling which is found in most infarct and ACS lesions.
Differences in Measuring Stenoses Between Technologies
Pathologic studies in patients dying after AMI or with sudden coronary death indicate that plaque rupture of a TCFA is seen in 2/3 to 3/4 of culprit thrombosed plaques [11]. Kolodgie et al. found in pressure fixed coronary arteries at autopsy, that asymptomatic TCFAs in general are not severely narrowed. Eighty percent occur in vessels with <75 % area stenosis which corresponds to <50 % diameter stenosis [12]. It should also be noted that pathology and IVUS measure narrowing differently than coronary angiography. The angiogram compares a narrowing to a proximal reference segment while area stenosis is measured by calculating the change in area at the site of narrowing to the external elastic membrane area. As lesions responsible for acute syndromes are usually positively remodeled [7] but not the proximal reference segment, area narrowing may overestimate stenosis severity relative to angiographic diameter stenosis. Thus, prior to MI or in patients with unstable symptoms, the plaque responsible for the subsequent event may be large but often exhibits <50 % diameter narrowing on angiography. These plaques are bulky but quiescent; most are TCFAs and positive remodeling usually preserves lumen diameter and luminal area.
Not all studies indicate that mild lesions precede ACS and even transmural MI. The angiographic study of Alderman et al. in 1993 appears to be contrary to the mild concept [13]. Five year angiographic follow up from participants in CASS (Coronary Artery Surgical Study) indicated that severe lesions (>80 % diameter narrowing on the first angiogram) were more likely to totally occlude on follow up study compared to less severe lesions. However, no clinical data were available to assess symptoms at the time of follow up. Furthermore, there were many more lesions that were milder or <80 % narrowed that occluded on the repeat study (71 % or 52 of 73) while only 29 % were >80 % initially (their qualitative analysis only categorized lesions into no narrowing, 5–49 %, 50–80 % and >80 % narrowed). In a subsequent publication from CASS, Ellis et al. found that the highest risk for subsequent anterior infarction (either transmural or non-transmural) was a severe stenosis (90–98 %) in the left anterior descending. However, angiographic follow up after the infarction was not available to confirm the severe stenosis as the culprit lesion [14]. Similarly, in the Program on the Surgical Control of the Hyperlipidemias (POSCH), Buchwald et al. concluded that severe lesions preceded most transmural or Q wave infarcts. Again, no follow up angiography was available to identify the culprit [15].
The last study of import comes from the Patients in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial. Mancini et al. analyzed 61 of 119 (56 %) of patients in the optimal medical therapy arm who subsequently had an infarct and a subsequent angiogram as well as other patients who had an ACS without infarction or just more angina requiring subsequent angiography[16]. These authors found for the entire group that lesions originally with <50 % diameter stenosis at baseline were responsible for only one third of events while the rest occurred in lesions with >50 % diameter stenosis at baseline. Yet, patients with MI were not analyzed separately and particularly in those with STEMI, baseline angiographic data were not reported. Thus, in the three studies mentioned above, 2 (Ellis and Buchwald) did not have follow up angiograms and 2 (Ellis and Mancini) included both STEMI and non STEMI cases in their analyses. We believe that because of these discrepancies, these data do not undermine the mild lesion argument.
Any follow up data that includes ACS patients that are not STEMI are also contaminated by the possible presence of supply/demand mismatch (Type 2 MI) as the mechanism for AMI [1]. Type 2 MI can occur in the presence of severe coronary disease although culprit lesions are not identified angiographically in nearly all of these patients. On the other hand, in Type I MI, single angiographic culprits were identified in 95 and 56 % of STEMI and NSTEMI patients, respectively [17].
Angiographic Narrowing in the Days to Weeks Prior to AMI and AMI Pathogenesis
Two studies have assessed angiographic narrowing in the days to weeks before STE MI. The earlier studies quoted above [2–6] did not compute angiographic stenosis data related to the timing of the baseline study prior to AMI. The largest of these two newer studies by Ojio et al. retrospectively assessed 40 patients with 2 angiograms before and after AMI onset similar to the analyses reported above [18]. However, when they analyzed the angiographic narrowing in 20 patients in whom the angiogram was obtained 3 ± 3 days before Q (n = 12) or non Q AMI (n = 8), the angiographic narrowing averaged 71 ± 12 % at the culprit site while it was 30 ± 18 % in the 20 control patients with a first angiogram that was 6–18 months before the subsequent AMI (see Fig. 2.2). They concluded that the lesion immediately before AMI is often severe. Likewise, in another small retrospective study, Zaman et al. found that lesions leading to STEMI were more often narrowed on the first angiogram if the clinical event was ≤3 months after the initial angiogram (n = 7) compared to >3 months (n = 34), 59 ± 31 % vs 36 ± 21 %, diameter stenosis respectively, p = 0.02 [19].
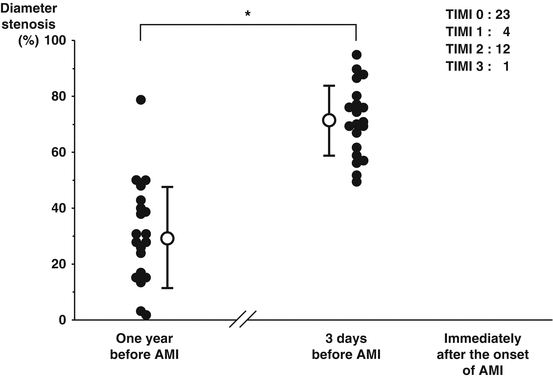

Fig. 2.2
Percent diameter stenosis of infarct related coronary stenosis 1 year before AMI, 3 days before onset, and immediately after onset of AMI (Reproduced from Ojio et al. [18], with permission of Wolters Kluwer health)
However, the question that must be asked in both of these studies is why were these patients studied right before the MI? The likely answer was that they were either symptomatic with new onset unstable angina and/ or a lesion had asymptomatically destabilized yet had not totally occluded prior to the acute clinical event. In support of that hypothesis was the fact that 70 % of patients in the group with an angiogram 3 days before AMI in the Ojio et al. study had angiographic evidence of an acute, complex culprit lesion as described originally by Ambrose et al. while these lesions were infrequent in the control group (10 %). These complex lesions indicate plaque disruption and/or intracoronary thrombus [20]. Thus, this group just happened to be studied immediately prior to the onset of either Q or non Q MI.
Growth of the plaque and narrowing of the lumen must occur at some point prior to the onset of AMI as the lesion progresses to total or near total occlusion at the time of the clinical event. Pathologically, autopsy studies indicate that the thrombosed lesion responsible for fatal MI or sudden coronary death contains both acute and healed thrombus [21, 22]. Multiple episodes of asymptomatic thrombus formation related to plaque disruptions or plaque hemorrhage usually precede the fatal event. These are the processes responsible for rapid progression of atherosclerotic lesions. Pathologic analysis of thrombectomy specimens at the time of primary PCI in ST elevation MI patients have also indicated that organized thrombus can be extracted in over 50 % of cases [23]. This also suggests some chronicity to the process. i.e. intracoronary thrombus formation does not automatically or immediately lead to a clinical event and the thrombus forms before the onset of symptoms in most patients with STEMI.
Thus, plaque instability and intraluminal thrombus formation must commonly precede the onset of infarction and total or near total coronary occlusion. This process is seen in both asymptomatic and symptomatic patients before infarction. If one considers unstable angina to be the forerunner to AMI particularly if untreated, plaque disruption and/or thrombus (a complex plaque) will be seen in >70 % of cases in the culprit vessel on coronary angiography [20]. Total occlusion in this setting is unusual but the culprit lesion has a severe diameter stenosis (>70 %) in nearly all cases [24]. These processes, we believe help to explain the findings of Ojio et al. and Zaman et al.
Angiographic Narrowing Immediately After Lysis or Thrombectomy
As mentioned earlier, ST elevation MI presents with total or near total coronary occlusion caused by intracoronary thrombus formation on a disrupted or eroded atherosclerotic plaque. Following successful opening of a totally occluded vessel with thrombolytic therapy or after mechanical thrombectomy, the culprit lesion is severely narrowed in nearly all vessels. This has been utilized as an argument favoring the concept that a severe lesion usually precedes MI. Thus, in 2007, Frobert et al. reported on 151 STEMI patients with spontaneous reflow or with immediate reflow after uncomplicated wiring of the lesion but before primary PCI. In 96 %, the underlying diameter stenosis was >50 % and in 66 %, it was >70 % [25]. Similar findings were also reported in 2009 by Manoharan et al. after mechanical thrombectomy in 102 STEMI patients. The underlying culprit lesion was severe in nearly all and was <50 % in only 11 % [26].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


