Previous studies have shown a poor correlation between angiographic assessment of stenosis grade (%) and its functional assessment by fractional flow reserve (FFR). This study aimed to investigate whether a more comprehensive evaluation of the coronary angiogram may contribute to a better identification of flow-limiting stenoses. Coronary angiograms of 1,350 patients (1,883 lesions) were retrospectively analyzed for stenosis grade (eyeballing, %) and matched with FFR values. Angiography-derived optimal cut-off values and intervals delineating the [90% sensitivity–90% specificity] range were 50.8% [42.5–65.0%] for the left main (LM), 62.2% [50.0–72.5%] for the proximal (prox)/mid left anterior descending (LAD) artery, 66.3% [57.5–77.5%] for the prox/mid right coronary artery (RCA), 70.5% [60.0–80.0%] for the prox left circumflex/first obtuse marginal (LCX/OM1), and 71.4% [62.5–82.5%] for the more distal segments. In patients with intermediate LAD lesions, 5 angiographic parameters were identified as independent predictors of flow limitation: (1) a 30–50% lesion prox to the lesion of interest, (2) lesion length >20 mm, (3) distal take-off of all diagonal branches ≥2 mm diameter, (4) “apical wrap” of LAD, and (5) collaterals to an occluded LCX/RCA. Based on these results, a risk score (P20-DAC 2 ) for prediction of flow limitation in intermediate LAD lesions was derived. In conclusion, a comprehensive evaluation of the coronary angiogram–in which besides stenosis grade also other lesion/vessel characteristics are evaluated–can lead to a more accurate identification of functionally significant coronary stenoses.
For more than 30 years, invasive coronary angiography has been used to define the presence and extent of obstructive coronary artery disease. The introduction of invasive fractional flow reserve (FFR) measurement represented a paradigm shift, moving the “gold standard” from a purely x-ray angiography–based diagnosis toward a more functional assessment of coronary lesions. Previous studies have shown a poor correlation between angiographic assessment of coronary lesion severity, as evaluated by “eye-balling” or quantitative coronary angiography (QCA), and its functional assessment by FFR. As a result, the capability of the conventional angiogram to identify functionally significant coronary lesions has been questioned. However, in daily practice, many percutaneous coronary intervention (PCI) operators still base their decisions on the visual evaluation of the coronary angiogram. Therefore, this study aimed to investigate whether the conventional coronary angiogram still has a role in the evaluation of intermediate coronary lesions and whether assessment of other angiographic parameters, besides stenosis severity, in intermediate left anterior descending (LAD) artery lesions may contribute to a more accurate identification of hemodynamically significant stenoses.
Methods
From July 2011 to June 2014, a total of 10,606 patients underwent a coronary angiography at our center. Of these, 1,483 patients had at least 1 coronary lesion evaluated by invasive FFR. For this study, patients with previous coronary artery bypass graft surgery were excluded, resulting in a study population of 1,350 patients. All clinical and procedural data were prospectively stored in a dedicated electronic database and were retrospectively analyzed in this study.
Coronary angiographies were performed through the radial or femoral access according to patient suitability and operator preference. In all patients, a 6Fr diagnostic or guiding catheter was used for the initial angiography. The angiographic recordings were stored in a digital archive (WEB 1000; Agfa-Gevaert NV, Mortsel, Belgium). All patients gave informed consent to the invasive procedure.
To obtain an unbiased assessment of all coronary lesions, 2 independent PCI operators retrospectively graded all coronary stenoses (eyeballing, %) in the 1,350 study patients. This assessment was performed blinded from all patient’s characteristics, including the original PCI operator’s estimation of lesion severity and FFR result—the mean of the 2 evaluations was used for subsequent analysis.
For patients with an LAD lesion, additional angiographic parameters were evaluated: (1) the presence of a mild-to-moderate lesion (30–50%) in the coronary segment prox to the evaluated lesion; (2) diffuse coronary artery disease—defined as LAD diameter <2 mm for at least 75% of the length of any segments prox to the lesion, at the site of the lesion, or distal to the lesion ; (3) severe calcification—defined as multiple persisting opacities of the coronary wall visible in >1 projection, surrounding the complete lumen of the coronary artery at the site of the lesion ; (4) lesion length >20 mm; (5) involvement of a bifurcation; (6) left dominant system; (7) localization in prox LAD—defined as the segment prox to the first major septal branch; (8) take-off of all diagonal branches ≥2-mm diameter distal to the site of lesion; (9) the presence of “apical wrap”—defined as an LAD that terminates >1/3 the way on the diaphragmatic surface; and (10) the presence of collaterals to a chronically occluded left circumflex (LCX) or right coronary artery (RCA).
After routine intracoronary administration of nitrates, FFR measurements were obtained under intravenous infusion of adenosine (140 μg/kg/min) by means of a pressure wire (St. Jude Medical Inc., Saint Paul, MN) advanced distally to the lesion. FFR was defined as the ratio of the mean coronary pressure distal to the stenosis to the pressure measured at the tip of the guiding catheter during steady-state hyperemia. Stenoses with FFR measurements ≤0.80 were considered ischemia-causing lesions.
Descriptive analysis was performed using mean ± SD for continuous variables and counts and percentages for categorical variables. The optimal cut-off value for visual lesion estimation was defined as the stenosis grade for which sensitivity equals specificity. A subgroup analysis was performed in patients with a 50–70% lesion in the prox/mid LAD to identify independent predictors of flow limitation (FFR ≤0.80). All variables with p ≤0.10 at univariate analysis were included in a stepwise multivariate logistic regression model, and a scoring system was derived based on the calculated odds ratio for significant predictors at multivariate analysis. All analyses were performed with SPSS software, v20 (SPSS, Chicago, IL).
Results
Baseline characteristics of the study population are summarized in Table 1 . The distribution of gender, age, and cardiovascular risk factors are comparable with those observed in the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) trials. Stable angina was the main indication for FFR evaluation (54.2%), followed by unstable angina/non–ST-elevation myocardial infarction (NSTEMI) in 25.0% of patients. A minority of patients (n = 145) had a non-invasive ischemia test before referral to invasive coronary angiography, resulting in 100 positive, 23 negative, and 22 non-conclusive tests.
| Variable | Study population (n = 1350) |
|---|---|
| Age (years) | 64.4 ± 10.6 |
| Male | 988 (73.2%) |
| Arterial hypertension | 848 (62.8%) |
| Hypercholesterolemia | 907 (67.2%) |
| Diabetes mellitus | 293 (21.7%) |
| Body mass index (kg/m 2 ) | 27.8 ± 10.4 |
| Current smoker | 379 (28.1%) |
| Positive family history of CAD | 609 (45.1%) |
| Previous myocardial infarction | 316 (23.4%) |
| Previous PCI | 580 (43.0%) |
| Left ventricular ejection fraction (%) | 50.7 ± 11.7 |
| Atrial fibrillation | 97 (7.2%) |
| Peripheral artery disease | 145 (10.7%) |
| Previous transient ischemic attack/stroke | 154 (11.4%) |
| Chronic kidney disease | 191 (14.1%) |
| Indication | |
| Stable angina pectoris | 732 (54.2%) |
| Unstable angina pectoris | 170 (12.6%) |
| NSTEMI | 167 (12.4%) |
| After STEMI | 140 (10.4%) |
| Cardiac arrest | 32 (2.4%) |
| Cardiomyopathy/heart failure | 42 (3.1%) |
| SVT/VT | 23 (1.7%) |
| Aortic valve disease | 31 (2.4%) |
| Mitral valve disease | 7 (0.5%) |
| Infective endocarditis | 4 (0.3%) |
| Aortic aneurysm | 2 (0.1%) |
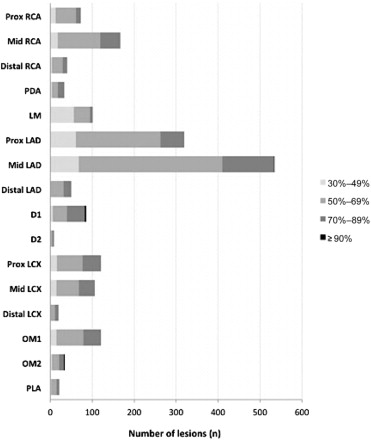
A total of 1,883 lesions were evaluated by FFR in 1,350 patients (mean of 1.39 lesions per patient). FFR was mainly measured in prox/mid LAD segments (n = 853), accounting for almost half of all FFR measurements. Other segments that were regularly evaluated by FFR were mid RCA (n = 167, 9.1%), prox LCX (n = 121, 6.6%), first obtuse marginal (OM1; n = 121, 6.6%), and left main (LM; n = 101, 5.5%; Figure 1 ).
The scatter plot diagrams in Figure 2 illustrate the correlation between stenosis grade (horizontal axis) and FFR result (vertical axis) for every single lesion evaluated by FFR—as grouped by LM, prox/mid LAD, prox LCX/OM1, prox/mid RCA, and all distal segments. Considering FFR as the “gold standard” for the identification of flow limitation (FFR ≤0.80, “condition” present) and stenosis grade (%) as the “test” to identify functionally significant lesions, the corresponding sensitivity and specificity of the “test” at different cut-off values were calculated.
Angiography-derived optimal cut-off values resulted to be 50.8% for the LM, 62.2% for the prox/mid LAD, 66.3% for the prox/mid RCA, 70.5% for the prox LCX/OM1, and 71.4% for the distal segments. The stenosis grade intervals delineating the [90% sensitivity–90% specificity] range were [42.5–65.0%] for the LM, [50.0–72.5%] for the prox/mid LAD, [57.5–77.5%] for the prox/mid RCA, [60.0–80.0%] for the LCX/OM1, and [62.5–82.5%] for the distal segments. In addition, the stenosis grade intervals delineating the [98% sensitivity-98% specificity] range were [35.0–72.5%] for the LM, [45.0–80.0%] for the prox/mid LAD, [52.5–82.5%] for the prox/mid RCA, [52.5–85.0%] for the prox LCX/OM1, and [55.0–87.5%] for the distal segments.
In patients with intermediate LAD lesions, a set of additional angiographic characteristics was evaluated. Univariate and multivariate analyses were performed to identify those variables associated with functionally significant stenoses (FFR ≤0.80). Five independent angiographic predictors of flow limitation were identified. Besides 2 lesion–related characteristics, i.e. lesion length >20 mm and a mild-to-moderate tandem lesion (30–50%) prox to the lesion of interest, 3 other factors related to the “supply area” of the diseased vessel were identified as independent predictors, i.e. distal take-off of all diagonal branches ≥2 mm, “apical wrap” of LAD, and collaterals to an occluded LCX or RCA ( Table 2 ).
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p value | Odds Ratio (95% CI) | p value | |
| Lesion characteristics | ||||
| Proximal segment 30-50% | 2.51 (1.72-3.65) | < 0.001 | 2.27 (1.50-3.43) | < 0.001 |
| Diffuse CAD | 1.92 (0.97-3.70) | 0.051 | 1.42 (0.55-2.38) | 0.623 |
| Severe calcification | 1.36 (0.93-1.99) | 0.114 | – | – |
| Length > 20 mm | 3.92 (2.76-5.58) | < 0.001 | 3.72 (2.60-5.33) | < 0.001 |
| Bifurcation | 1.39 (0.86-2.24) | 0.180 | – | – |
| Supply area | ||||
| Left dominance | 0.89 (0.51-1.54) | 0.679 | – | – |
| Proximal LAD | 1.37 (0.98-1.93) | 0.070 | 1.22 (0.82-1.81) | 0.253 |
| Distal take-off of all diagonals ∗ | 1.83 (1.29-2.59) | 0.002 | 1.54 (1.04-2.30) | 0.032 |
| Apical wrap | 3.16 (2.03-4.89) | < 0.001 | 3.94 (2.50-6.21) | < 0.001 |
| Collaterals to RCA/LCX | 6.08 (3.20-11.54) | < 0.001 | 8.18 (4.25-14.75) | < 0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


