Hepatomegaly
Peripheral edema
Ascites
Left parasternal heave
Systolic murmur in second left intercostals space
Loud P2 and split second heart sound
Third and fourth heart sound
Prominent jugular pulse wave
Holosystolic murmur in fourth right intercostal space
Indicative of tricuspid regurgitation
Signs and symptoms of associated diseases
When planning surgery in a patient with RV dysfunction, the perioperative evaluation should include thorough risk assessment on the type of surgery, functional status, severity of disease, and comorbidities. The latter mainly comprise renal, hepatic, and coagulation derangement, which are common in patients with RV failure, and hence, they have to be optimized preoperatively. This is critical when high-risk surgical procedure is scheduled: cardiac surgery (when rapid blood loss is evident and systemic inflammatory response is triggered), thoracic surgery (when loss of lung parenchyma is anticipated), laparoscopic surgery (when venous air embolism from carbon dioxide may occur), orthopedic surgery (when fat or cement embolism is possible), etc. Treating RV failure by optimizing preload, afterload, and contractility in the perioperative period is crucial to prevent a dismal outcome. This includes fundamental measures such as maintenance of normal sinus rhythm or atrioventricular synchronicity, adequate ventilation, temperature, acid-base balance, and prevention of coagulopathy. Van Meter suggested that RV failure should be treated perioperatively mainly by “avoidance” and does not always require aggressive treatment [1].
As far as preoperative medication is concerned, these patients are typically treated with intensive diuretic therapy, b-blockers, and ACE inhibitors. Diuretic treatment should be discontinued on the day of surgery, due to hypovolemia-induced hypotension after induction of general anesthesia. There is general consensus that treatment with b-blockers should be continued perioperatively in order to avoid rebound tachycardia and hypertension. Moreover, ACE inhibitors should also be administered as sudden withdrawal could result in exacerbation of heart failure [2]. It has to be emphasized that preoperative anxiolytic medication results in a decrease of endogenous sympathetic tone and should, therefore, be administered judiciously. Furthermore, oversedation could lead to hypoventilation, which in turn results in hypoxia, acidosis, and an increase in pulmonary vascular resistance (PVR). Since there is a minimal physiological reserve in this high-risk group of patients, even minor alterations could precipitate major deterioration.
11.2 Intraoperative Anesthetic Management
Anesthetic management in patients with RV dysfunction is quite challenging. Ideally, anesthesia should be carried out by an anesthesiologist expert in cardiovascular hemodynamics with experience in managing patients with right heart dysfunction. Concurrent intraoperative use of pulmonary artery catheter (PAC) and transesophageal echocardiography (TEE) is of paramount importance. Right heart catheterization provides continuous monitoring of the RV and pulmonary artery pressures as well as RV cardiac output, while TEE is helpful in evaluating volume status and response to therapy. Care must be taken to optimize RV filling by controlling heart rate and rhythm. Special attention should be given to the induction of anesthesia, when acute hypotension and fall in cardiac preload may present as a result of anesthetic-induced venodilation. This should be expected, because of the relative intravascular hypovolemia at baseline caused by diuretics exacerbated by any period of preanesthetic fasting. It should be noted that poor RV systolic function predisposes to acute deterioration following anesthesia induction, despite relatively normal vital signs preoperatively. Since these patients often have a cardiac resynchronization therapy (CRT) as well as implantable cardioverter-defibrillator (ICD), special measures are warranted preinduction. The rate responsive mode, antitachycardia mode, and defibrillator should be disabled in order to decrease the incidence of improper sensing or pacing secondary to electrocautery and electromagnetic interference in the operating room. Since ventricular arrhythmias which may be resistant to drug therapy are sometimes evident during induction, external shock paddles should be placed beforehand.
11.2.1 Anesthesia Induction
Upon arrival in the operating room, oxygen via a face mask should be administered. Standard monitoring [5-lead electrocardiography (ECG), pulse oximetry, capnography, and core/peripheral temperature recording] and invasive arterial blood pressure monitoring are mandatory. A large-bore peripheral venous access (14 or 16G) is useful for rapid fluid infusion. A multi-lumen central venous catheter is also mandatory. Initiation of low-dose inotropic or vasopressor therapy prior to anesthetic induction may be warranted, so as to provide a baseline support facilitating titration in the event of hemodynamic deterioration. By all means, it has to be noted that uncompensated vasodilatation or myocardial depression caused by anesthetics and mechanical ventilation may be responsible for acute RV failure. We advocate initiation of a low-dose norepinephrine just before induction, so as to compensate for the drop of catecholamines due to decreased sympathetic output caused by anesthesia [3, 4]. Administration of vasoactive agents should be adjusted to maintain optimal hemodynamics and support adequate depth of anesthesia.
Regarding anesthesia induction, a balance of small incremental doses of benzodiazepine, fentanyl, propofol, or etomidate could be administered. Etomidate is a suitable induction agent for patients with RV dysfunction and pulmonary arterial hypertension (PAH), since it has minimal effect on systemic vascular resistance (SVR), PVR, or myocardial contractility. It should be emphasized that there is a slow circulation time and reduced volume of distribution for these drugs in patients with heart failure [5]. Target-controlled infusion (TCI) comprising combination of propofol and remifentanil with low targets allowing adequate time to assess the effect represents a valuable strategy. The depth of anesthesia is recommended to be monitored by processed electroencephalogram (BIS). Muscle relaxation can be achieved using rocuronium or cisatracurium. Regarding effects of mechanical ventilation, the institution of positive-pressure ventilation impedes systemic venous return, leading to closure of small pulmonary arteries in hypovolemic patients, and abruptly increases PVR and, thus, RV afterload. Hence, once muscle relaxation has been achieved, overventilation should be avoided as it compromises venous return and induces hypocarbia; this may decrease circulating catecholamine levels and aggravate hypotension [6].
11.2.2 Maintenance of Anesthesia
Regarding maintenance of anesthesia, various techniques utilizing inhaled or intravenous agents are in clinical practice as well as combinations of both so as to minimize the adverse effects of any single agent. Use of TCI-adjusted short-acting opioid, such as remifentanil, enables titration according to the level of surgical stimulation, without impeding recovery. This also provides stable intraoperative hemodynamics as well as adequate analgesia. Modern inhaled agents (isoflurane, sevoflurane, and desflurane) have minimal effect on myocardial contractility and on PVR at clinically relevant doses, than old agents, such as halothane [7]. Adequate suppression of neuromuscular activity intraoperatively maintains neuromuscular block while lowers VO2 and, hence, decreases lactate production [8]. When cardiopulmonary bypass is used, such as in cardiac surgery, maintenance of anesthesia is achieved not only with inhaled but also with intravenous continuous propofol administration. This results in cerebral metabolic rate reduction, which may contribute to decreased incidence of cerebrovascular events [9].
11.2.3 Intraoperative Monitoring
Intraoperative monitoring of patients with RV dysfunction follows the same principles that apply to heart failure patients. As discussed previously, the 5-lead ECG is mandatory. Invasive hemodynamic monitoring with PAC offers recording of pulmonary artery pressures, calculation of PVR, and continuous RV cardiac output. It also facilitates goal-directed therapy of PAH-induced RV dysfunction with calculation of the transpulmonary pressure gradient [mean pulmonary artery pressure minus pulmonary capillary wedge pressure]. When exceeding normal values (7–8 mmHg), it provides an indication for initiation of pulmonary vasodilatory agents. This facilitates weaning from cardiopulmonary bypass that is demanding in these patients. Mixed venous oxygen saturation measurement is valuable, since it represents a reliable index for adequacy of tissue perfusion in heart failure patients. Moreover, cerebral oximetry is a useful tool to monitor adequate global perfusion, since it is affected from several parameters, like cardiac output, mean blood pressure (volume status, inotropic support), hematocrit, oxygenation, and integrity of microcirculation [10, 11]. Intraoperative TEE assessment of the RV is invaluable in guiding fluid and inotropic/vasoactive drug management as well as successfully weaning off cardiopulmonary bypass [12].
11.2.4 Hemodynamic Optimization
Principles in perioperative management of patients with RV dysfunction include (1) preservation of coronary perfusion through high mean arterial pressure; (2) RV preload optimization, which comprises intravenous fluids administration when central venous pressure is less than 10 mmHg up to the point that an increase in central venous pressure does not result in concomitant cardiac output increase; (3) RV afterload reduction by decreasing PVR; and (4) pulmonary vasodilation through ventilation with high inspired oxygen concentrations, appropriate tidal volume, and optimal positive end-expiratory pressure (PEEP) (Fig. 11.1).
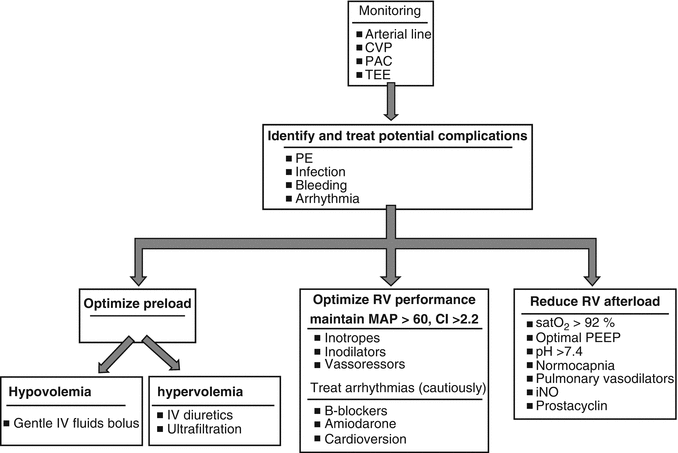

Fig. 11.1




Perioperative management algorithm of RV dysfunction related to PAH (Adapted from McGlothlin et al. [13]). (CI cardiac index, CVP central venous pressure, iNO inhaled nitric oxide, IV intravenous, MAP mean arterial pressure, PAC pulmonary artery catheter, PE pulmonary embolism, PEEP positive end-expiratory pressure, RV right ventricle, TEE transesophageal echocardiography)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


