CHAPTER 10 Alfred J. Albano, MD, Zachary Camann, MD, and Michael England, MD With the prevalence of atrial fibrillation (AF) predicted to exceed 12 million cases in the United States by 2030, electrical cardioversion and AF ablation procedures will likely comprise a major portion of procedural volume in most labs.1 Clinical electrophysiology has undergone a dramatic transformation over the past several decades. Once limited to simple diagnostic procedures, the electrophysiology lab (EPL) has evolved into an interventional suite where intricate and complex therapies are performed on a daily basis. Radiofrequency ablation for AF is among the most technically demanding procedures now performed in the EPL. Electrical cardioversions have become a daily fixture in the lab. To meet the clinical demands of these procedures, the need for anesthesiology expertise in the EPL is instrumental. This chapter is devoted to summarizing published current best practices in the delivery of anesthesia care to patients being treated with direct current cardioversion or radiofrequency ablation for AF. A standard set of information should be collected and documented during the preanesthesia evaluation (Table 10.1). This initial evaluation should always begin with a comprehensive history and physical examination. A current and detailed note including the indication for the procedure should be completed by the electrophysiology team and be available at the time of evaluation by the anesthesia care team (ACT). The history-taking process should be devoted to eliciting a complete past medical history, obtaining an accurate medication list (including medications taken and not to be taken the day of procedure), confirming allergies (including latex or heparin) and NPO status. Timing of the last β-blocker dose, use of antiarrhythmic medications, and anticoagulation status require special attention. Patients should also be asked about prior adverse anesthetic experiences and/or difficulties with intubation or malignant hyperthermia. A history of postoperative nausea and vomiting (PONV) as well as other risk factors should routinely be elicited, so prophylactic antiemetics can be considered.2
Anesthesia Care for the Atrial Fibrillation Patient: Cardioversion and AF Ablation
PREANESTHESIA EVALUATION
Patient History
ASA Guidelines for Documentation of Care |
Patient History |
• Patient and procedure identification • Verification of admission status • Medical history • Anesthetic history • Medical/allergy history • NPO status |
Physical Exam |
• Vital signs • Airway evaluation |
Objective Data |
• Laboratories • Chest radiograph • 12-lead electrocardiogram • Echocardiogram |
Formulation of Anesthetic Plan |
• Documentation of risks • Informed consent • Documentation of anesthetic technique |
Premedication |
• Prophylactic antibiotics • Venous thromboembolism prophylaxis • Prophylactic antiemetics |
A comprehensive understanding of patients’ comorbidities is critical to ensure appropriate periprocedural management and minimize complications. In the unusual circumstance that objective data on cardiac function is unavailable, this can be grossly assessed by asking about functional capacity. The ability to climb a flight of stairs without dyspnea corresponds to approximately 4 METS. Patients who smoke or have pulmonary disease including obstructive sleep apnea are at higher risk for respiratory complications. The “STOP-Bang” questionnaire (loud snoring, tiredness during the daytime, observed apneas, high blood pressure, body mass index (BMI) > 35 kg/m2, over 50 years of age, neck circumference > 40 cm, and male gender) is a simple tool that can be used to quickly assess for underlying obstructive sleep apnea (OSA).3 Those with renal or hepatic dysfunction may require medication dosage adjustments and may be precluded from receiving certain medications. Type 2 diabetic patients should be counseled not to take their morning insulin or oral hypoglycemic agents on the day of the procedure and will need to have their glucose monitored and controlled if necessary with short-acting insulin. Type 1 diabetics may need to take one-third to one-half of their morning intermediate or long-acting insulin, as diabetic ketoacidosis may develop if medication is held. They should be educated on recognizing signs and symptoms of hypoglycemia that may occur prior to entering a healthcare facility. If that occurs, oral consumption of a clear liquid glucose-containing solution (50–100 mL) may be necessary. Medical consultations may be necessary to assist in the risk stratification and medical optimization of particularly challenging patients with multiple comorbidities. Subspecialty consultants should help assess whether the patient’s comorbidities are optimally treated and recommend specific therapies to decrease the risk of periprocedural complications.
Physical Examination
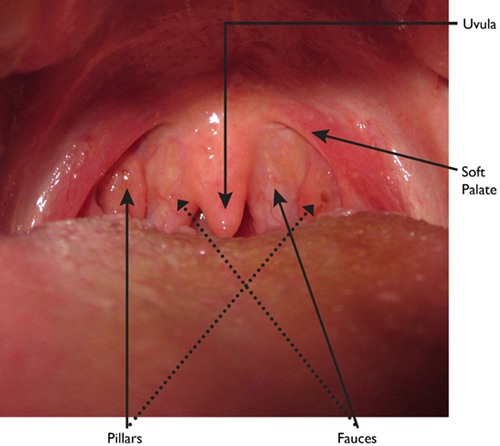
In addition to the patient history, a thorough physical examination should be performed, noting vital signs (including bilateral upper extremity blood pressures) and evaluating the airway. Height, weight, and BMI should be documented. The Mallampati classification system is commonly used among anesthesiologists to predict the ease of intubation. This was originally described in 1985 as a three-class system but then modified by Samsoon and Young in 1987 to include four anatomical landmarks: the soft palate, fauces, uvula, and pillars (Figure 10.1). A patient with a class I oropharynx has all four landmarks visible. In class II, the soft palate, fauces, and uvula are visible. In class III, only the soft palate and base of the uvula can be seen, whereas in class IV the entire soft palate is not visible.4,5 A favorable modified Mallampati class (class I) does not always predict an easy intubation, nor is an unfavorable modified Mallampati class (class IV) always predictive of difficulty. In fact, a recent meta-analysis determined that the Mallampati test itself had poor to moderate discriminative power when used alone in this regard.6 However, this method of airway evaluation is commonly and widely used as an initial tool in alerting the ACT that there might be an airway issue.
Anatomy of the posterior oropharynx.
The value of the Mallampati score can be improved by considering it in conjunction with other predictors of difficult intubation. These factors include: small mouth opening (a narrow inter-incisor distance), limited jaw mobility (an inability to push the lower jaw forward over the upper jaw), limited head extension, and a thyromental distance of less than 7 cm.7 Poor dentition can be a risk factor for dental damage during laryngoscopy, and certain patterns of dentition such as overhanging central incisors can make intubation challenging. This is especially important if the location of the procedure is “off site” as there may be a delay in getting assistance in a timely fashion. The American Society for Anesthesiology (ASA) has standardized the algorithm for managing anticipated and unanticipated difficult airways.8
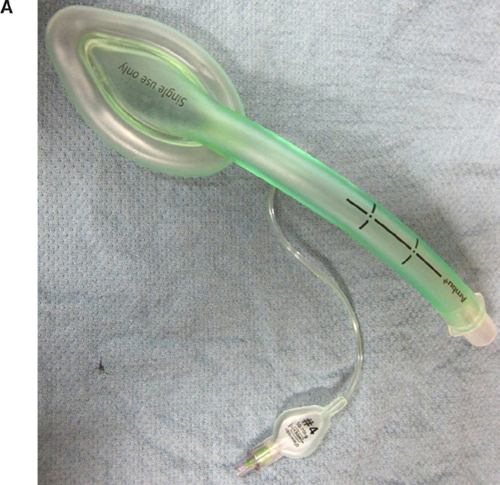
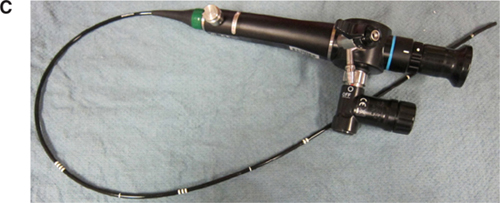
The risk factors that predict difficult intubation are different from those that predict difficult ventilation with a face mask. Predictors of difficult mask ventilation include obesity, increased neck circumference, advanced age, upper airway obstruction (as in sleep apnea), limited mandibular protrusion, and the presence of facial hair. A laryngeal mask airway (LMA) is an invaluable tool in the anesthesiologist’s airway armamentarium for both elective and rescue use in a situation where the patient cannot be intubated or ventilated. Aids to intubation should be readily available and close at hand during any induction of anesthesia so the anesthesiologist may be prepared for any complications that may ensue. In addition to an LMA, the use of an oro- or nasopharyngeal airway, a video laryngoscope [either disposable units made by AIRTRAC® and King Systems or reusable devices made by Verathon® (Glidescope), Teleflex® (McGrath), and Storz® (C-MAC)], or a flexible fiberoptic bronchoscope may prove invaluable in the setting of a difficult airway (Figure 10.2). Being well prepared is the key to avoiding an airway disaster.
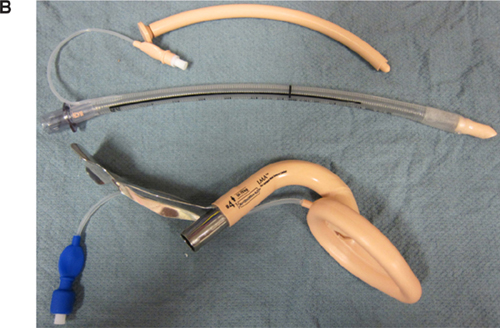
Intubation aids for challenging airways. A: Laryngeal Mask Airway. B: Intubation Tray (top to bottom: Nasopharyngeal Airway, Endotracheal Tube, and Laryngeal Mask Airway). C: Flexible Fiberoptic Scope. D: Video Laryngoscope.
An important part of the preanesthesia evaluation is the determination of the ASA physical status. The ASA physical status classification was introduced in 1940 as a global assessment of the patient’s state of health (Table 10.2). The values range from 1 to 6, with 1 denoting a healthy patient and 6 indicating a brain-dead organ donor. Importantly, an ASA class of 5 describes a moribund patient who is not expected to survive with or without the procedure. An “E” designation may be added for emergency cases whereby delaying the case (in cases of a recent meal or liquids of any quantity) would not be appropriate, especially in a patient with unstable hemodynamics. The utility of the ASA physical status is that it clearly communicates an anesthesiologist’s prediction of morbidity and mortality based on a comprehensive evaluation of the patient’s current condition. This classification can also be used to stratify patients for outcomes-related data analyses.
Table 10.2 | |
Physical Status | Description |
1 | Normal, healthy patient |
2 | Mild systemic disease without functional limitation |
3 | Severe systemic disease with functional limitation |
4 | Life-threatening, severe systemic disease |
5 | Moribund, not expected to survive operation |
6 | Brain-dead organ donor |
E | Emergency operation |
Review of Diagnostic Studies
Objective laboratory data, radiographic studies, prior cardiac testing, previous electrophysiology procedures, and prior surgeries (noting any previous difficulty by a former ACT) should also be carefully reviewed. Special attention should be devoted to the patient’s renal and hepatic function, serial coagulation studies, the baseline 12-lead electrocardiogram (ECG), and recent echocardiography exams (including transesophageal studies) if performed. In accordance with the 2011 ACC/AHA guidelines, patients with AF of an unknown duration or > 48 hours undergoing direct current cardioversion should have weekly INRs ≥2 for 3 weeks prior to cardioversion.9 Alternatively, a TEE excluding left atrial appendage thrombus can be performed to eliminate the need for 3 weeks of anticoagulation or those patients having difficulty achieving 3 weeks of weekly therapeutic INRs. In patients with an elevated CHA2DS2-VASc score, particularly those with heart failure and diabetes, there is a higher risk of stroke even if cardioversion is performed within 48 hours.10 The use of TEE should therefore be considered in this high-risk patient population. Patients with a history of atrial thrombus should be anticoagulated for 4 to 6 weeks and have demonstrable resolution of clot on a repeat TEE before attempting cardioversion. The ECG should be carefully analyzed for the presence of conduction system disease, ST-T wave abnormalities, and QTc prolongation. A chest radiograph should be obtained to assess for occult pulmonary disease, evaluate positioning of any devices, and establish a baseline measurement for the cardiac silhouette. When a patient’s functional capacity is ambiguous, a direct assessment of LV function using transthoracic or transesophageal echocardiography should be performed if such testing has not been done already.
Formulation of the Anesthetic Plan
The goal of anesthesia is to maintain patient comfort and a quiet procedural field through a combination of agents producing anxiolysis, amnesia, and analgesia. The extent of the procedure and the physical status of the patient should always be taken into consideration when formulating the anesthetic plan. In addition, it is important to note that levels of sedation (Table 10.3) are a continuum, and inadvertent administration of an excessive dose of medication may risk airway compromise. Thus, any plan to sedate a patient must include contingencies for intensive and advanced airway management should general anesthesia become necessary. The North American Society of Pacing and Electrophysiology consensus document states that anesthesia personnel should be involved in all EPL cases requiring the use of deep sedation or general anesthesia with or without an artificial airway.11 According to the ASA guidelines, nonanesthesia practitioners who administer deep sedation must be trained in appropriate rescue airway techniques.12 The use of deep sedation in many EPLs without the oversight of an ACT is an ongoing safety concern.13
| Minimal Sedation (Anxiolysis) | Moderate Sedation (Analgesia) | Deep Sedation (Analgesia) | General Anesthesia |
Responsiveness | Normal response to verbal stimulation | Purposeful response to verbal or tactile stimulation | Purposeful response following repeated or painful stimulation | Unarousable even with painful stimulus |
Airway | Unaffected | No intervention required | Intervention may be required | Intervention often required |
Spontaneous Ventilation | Unaffected | Adequate | May be inadequate | Frequently inadequate |
Cardiovascular Function | Unaffected | Usually maintained | Usually maintained | May be impaired |
PROCEDURAL ANESTHESIA
Direct Current Cardioversion
General Concepts of Direct Current Cardioversion
Closed-chested defibrillation was first introduced in the 1950s but not popularized until the 1960s when Lown and colleagues introduced direct current cardioversion for the treatment of AF.14 The precise mechanism by which electrical cardioversion restores normal sinus rhythm is not clear, although Zipes argues that depolarization of a critical mass of myocardium interrupts fibrillatory propagation, terminates reentry, and resets the rhythm.15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree