Figure 4–1.
(a) Diagram illustrating the topography of the specialised conduction system (red) of the heart; (b) view of the right atrium: the sinus node is located at the root of the superior vena cava, lying over the crista terminalis, and the AV node within the triangle of Koch. Dotted lines depict internodal pathways and left bundle branch. (c) Diagram illustrating the internodal pathways (A anterior, M middle, P posterior) between the sinus node (SN) and the AV node (AVN) and the interatrial connection through the so-called Backman’s bundle (BB). FO fossa ovalis, MS membranous septum, HB His bundle, LBB left bundle branch, RBB right bundle branch
Normal Anatomy
Sinus Node: Location, Anatomy and Histology
Discovered by Keith and Flack a century ago [1], the sinus node (the cardiac pace-maker) was illustrated as lying in the terminal groove (sulcus terminalis), in the lateral part of the junction between the superior vena cava and the right atrium (see Fig. 4.1). The original description reports that “there is a remarkable remnant of primitive fibres persisting at the sino–auricular junction in all the mammalian hearts examined. These fibers are in close connection with the vagus and the sympathetic nerves, and have a special arterial supply; in them the dominating rhythm of the heart is believed to normally arise” [1].
This lateral position was endorsed by Koch [2] and by most subsequent investigators [3–5]. A horse-shoe arrangement with the node situated anteriorly and draped over the crest of the atrial appendage as described by Hudson [6] is found in approximately 10 % of hearts [7].
The shape of the node, more commonly, is like a tadpole with a head section situated antero-superiorly and a tapering tail that extends for a variable distance inferiorly toward the entrance of the inferior vena cava (see Fig. 4.1) [2, 8]. In the subepicardium, the long axis of the node is parallel to the terminal groove, but the body and tail then penetrate intramyocardially towards the subendocardium. Thus, the fatty tissues of the terminal groove serves as the epicardial landmark whereas the terminal crest (crista terminalis) in the antero-lateral quadrant of the entrance of the superior vena cava is the endocardial landmark for the nodal head. The nodal body and tail lie in the terminal crest and are at varying depths from the endocardium [8]. In the adult the length of the nodal body is approximately 1–2 cm but the tail portion can extend considerably longer.
The artery supplying the node is a branch from the proximal right coronary artery in 55 % of hearts and from the left circumflex coronary artery in the remainder [7]. The nodal artery approaches the node from anteriorly in majority of hearts but can also approach from posteriorly or form an arterial circle around the cavo-atrial junction [9]. Typically, the nodal artery passes centrally through the length of the nodal body.
The node is a specialised muscular structure composed mainly of small, interlacing myocytes of no definite orientation within a background of extracellular matrix, surrounded by para-symphatetic ganglionated plexus accounting for nerve supply [10]. On histology, the node appears as a dense aggregation and the specialised myocytes (P cells) appear less darkly stained than the neighboring working atrial myocardium (Fig. 4.2). These P cells have pacemaker activity due to spontaneous depolarization. At electron microscopy, P cells typically occur in small clusters of 3 or 4, surrounded by a basement membrane and contain very simple intercellular junctions (Fig. 4.3). The intercellular junctions of the P cells are composed mainly of undifferentiated regions, with only a few desmosomes and even more rarely, a very small gap (nexus).
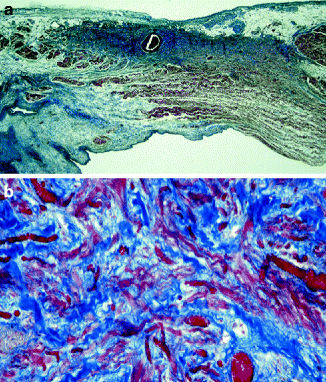
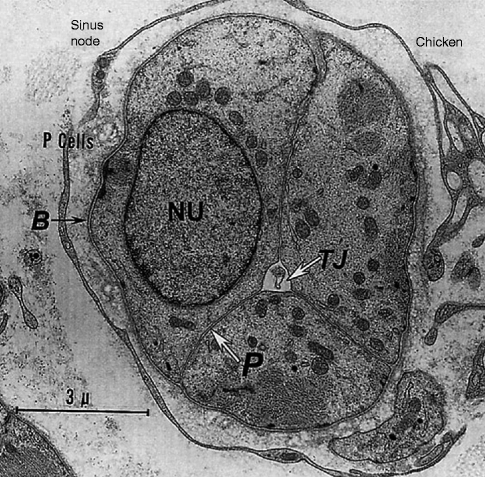

Figure 4–2.
(a) Longitudinal section of the sulcus and crista terminalis: the sinus node is located sub-epicardially and centered by the sinus node artery. Note the abundant extracellular matrix (Heidenhain trichrome ×5); (b) at higher magnification, the small, interlacing pale myocytes with P and T cells are visible (Heidenhain trichrome ×40)

Figure 4–3.
Clusters of P-cells in the chicken sinus node. Note a basement membrane (B) surrounding the entire P-cell cluster. Junctions between the three P cells here are composed entirely of “undifferentiated regions,” with simple apposition of plasma membranes (P) at a constant distance from each other (TJ triadic junction, NU nucleus) (From James [10]. Reprinted with permission from Elsevier Limited)
However, the nodal margins may be discrete with fibrous separation from atrial myocardium or interdigitate through a transitional zone. In the latter, prongs of nodal (P) and transitional (T) cells extend into the atrial myocardium but actual cell-to-cell contact is uncertain. Prongs radiating from the nodal body are common [8]. Occasional prongs can be found extending toward the wall of the superior vena cava. In some hearts, the distal part of the nodal tail appears as clusters of specialised myocytes amongst fibrofatty tissues and atrial myocytes in the subendocardium [8].
Internodal and Interatrial Myocardium
The transmission of the cardiac impulse from the sinus node to the AV node is often depicted as through three internodal tracts in Cardiology texts. These are portrayed as cable-like structures that pass anteriorly, medially and posteriorly in the right atrium (Fig. 4.1b, c). However, light microscopy and electron microscopy examinations have not revealed discrete specialised bundles in the atrial walls that could satisfy the criteria of conduction tissue tracts. Apart from the occasional caudal extension of the sinus node into the crista terminalis, there are no histologically recognizable specialised pathways. This is also true for the interatrial conduction through the so-called Backman’s bundle [11]. Instead, the walls of the atria are made up of broad bands of working myocardium that are separated by orifices of the veins, foramen ovale, and the AV valves. Parts of the wall, for example the crista terminalis and the anterior rim around the foramen ovale, show a better alignment of the myocytes than other parts, allowing preferential propagation of the cardiac impulse. Thus, the spread of excitation from pacemaker to AV node as well as to the left atrium is along broad wavefronts in these muscular bands. Through its transitional cell zone, the AV node acts as the ‘receiver’ that then channels the impulse to the ventricles via the specialised conduction bundle and bundle branches.
AV Conduction System: Location, Anatomy and Histology
The pioneering work of Tawara [12] a century ago likened the AV system to a tree, with its roots in the atrial septum, and its branches ramifying within the ventricles. He recognized a collection of histologically distinct cells at the base of the atrial septum that he termed the “knoten”, and that has subsequently become known as the AV node.
Being the atrial component of the AV conduction system, the AV node receives, slows down and conveys atrial impulses to the ventricles. It is an interatrial structure located on the right side of the central fibrous body and when considered from the right atrial aspect it is situated within the triangle of Koch (see Fig. 4.1). The triangle described by Koch [2] is bordered anteriorly by the ‘annulus’ of the septal leaflet of the tricuspid valve, posteriorly by the tendon of Todaro that runs within the sinus septum (Eustachian ridge or crista dividens), and inferiorly by the orifice of the coronary sinus and the atrial vestibule (see Fig. 4.1). The vestibule is recognised by arrhythmologists as the so-called ‘septal isthmus’. This is the target for ablating the slow pathway in patients with AV nodal reentrant tachycardia [13]. The central fibrous body itself is comprised of a thickened area of fibrous continuity between the leaflets of the mitral and aortic valves, termed the right fibrous trigone (Fig. 4.4), together with the membranous component of the cardiac septum. The tendon of Todaro inserts into the central fibrous body that lies at the apex of the triangle (Fig. 4.5) [14]. The ‘annulus’ of the septal leaflet of the tricuspid valve crosses the membranous septum (Fig. 4.6a).
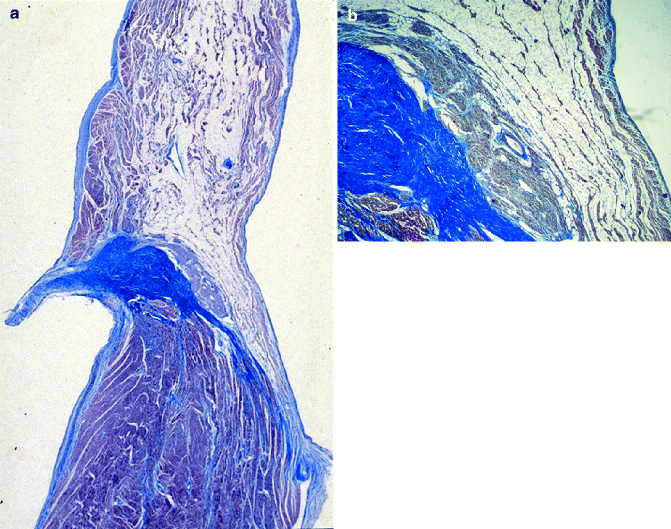
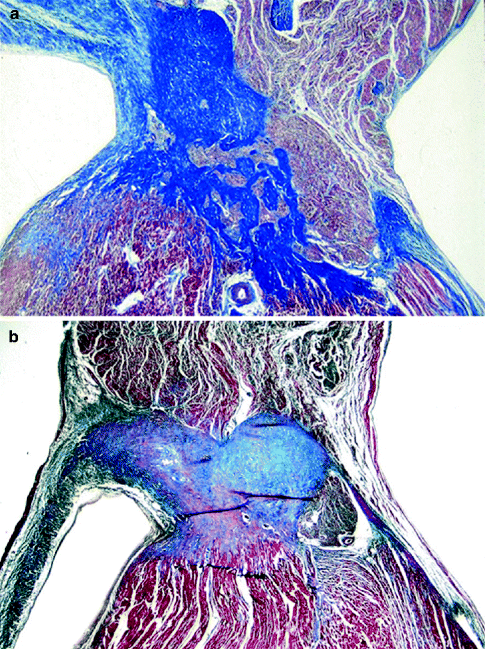
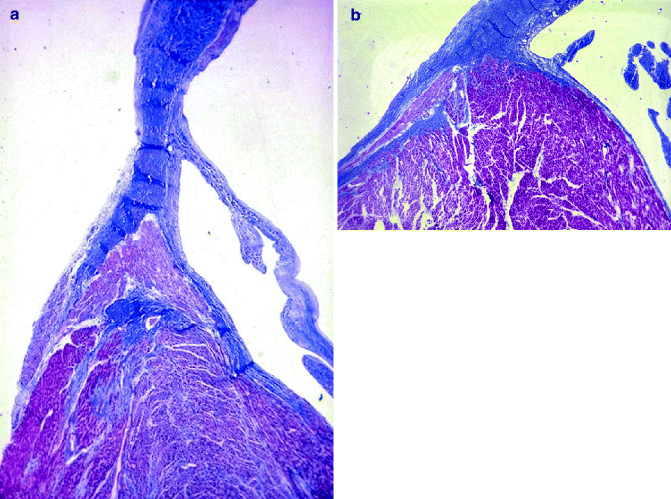

Figure 4–4.
(a) The AV node is located on the right side of the central fibrous body, which extends to the fibrous mitro-aortic continuity (Heidenhain trichrome ×3); (b) close-up of the AV node, with compact and transitional zones, centered by the AV nodal artery (Heidenhain trichrome ×12)

Figure 4–5.
(a) Penetrating AV bundle: note on the top the tendon of Todaro, approximating the central fibrous body (Heidenhain trichrome ×12); (b) common AV bundle running within the fibrous body on the right side and surrounded by a fibrous sheat (Heidenhain trichrome ×12)

Figure 4–6.
(a) Bifurcating bundle astride the ventricular septal crest, underneath the membranous septum: note the insertion of the septal leaflet of the tricuspid valve dividing the membranous septum in interventricular and AV components (Heidenhain trichrome ×4); (b) Course of the bifurcating bundle on the left side of the ventricular septal crest: note the insulation of the bundle by fibrous tissue and the intramyocardial course of the proximal right bundle branch (Heidenhain trichrome ×8)
In the original description by Tawara, it is reported that “the system is a closed muscle bundle that resembles a tree, having a beginning, or root, and branches… The system connects with the ordinary ventricular musculature for the first time at the terminal ramifications” [12]. Moreover, in 1893 His was the first to observe the bundle as follow: “ I have succeeded in finding a muscle bundle which unites the auricular and ventricular septal walls…The bundle arises from the posterior wall of the right auricle near the auricular septum, in the atrioventricular groove; attaches itself along the upper margin of the ventricular septal muscle…proceeds on top of this toward the frontal until near the aorta it forks itself into a right and left limb” [15].
The compact node, approximately 5 mm long, 5 mm wide and 0.8 mm thick in adults [16], is adjacent to the central fibrous body on the right side but is uninsulated by fibrous tissue on its other sides, allowing contiguity with atrial myocardium (see Fig. 4.4). Owing to the lower level of attachment of the tricuspid valve relative to the mitral valve, the AV node ‘leans’ toward the right atrial side and is a few millimetres far from the endocardium (see Fig. 4.4).
From the node extends the AV bundle of His that passes through the fibrous core of the central fibrous body (see Fig. 4.5). The bundle veers leftward as it penetrates the central fibrous body, taking it away from the right atrial endocardium and toward the ventricular septum. In majority of hearts it emerges to the left of the ventricular septal crest but is insulated from ventricular myocardium by fibrous tissue and from atrial myocardium by the membranous septum itself (Fig. 4.6b). Viewed from the left ventricle, the landmark for the AV bundle is the area of fibrous continuity between aortic and mitral valves that is adjacent to the membranous septum. Viewed from the aorta, the interleaflet fibrous triangle between the right and the non-coronary sinuses adjoins the membranous septum and the AV bundle passes beneath that part of the septum (Fig. 4.7).
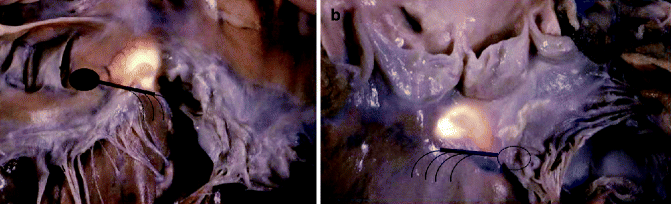

Figure 4–7.
The “core” of the heart in correspondence of the membranous septum, where the specialised AV junction is located (a, right side view; b, left side view). The landmark of the AV bundle from the left side is the continuity between the aortic and mitral valve, adjacent to the membranous septum, which is located underneath the interleaflet triangle between the right and posterior non-coronary cusps
Progressing forward, the AV bundle divides into left and right bundle branches, still en-sheathed by fibrous tissue until the bundle branches have descended approximately half-way down the septum. Descending in the subendocardium, the left bundle branch fans out into interconnecting fascicles as depicted in the original drawings by Tawara [12]. The fascicles then ramify into thinner and thinner strands toward the apex. Sometimes its proximal subendocardial course is visible due to the glistening sheen of its fibrous sheath. Owing to its fan-shape, its proximal portion is considerably more extensive than that of the right bundle branch. The right bundle branch, a cord-like structure, is a direct continuation of the AV bundle. In majority of hearts, since the AV bundle is usually to the left of the ventricular septal crest instead of being astride the crest, the right bundle branch passes through the septal myocardium before reaching the subendocardium of the right side of the ventricular septum (Fig. 4.6). The anatomical landmark for its emergence is the base of the medial papillary muscle (Lancisi muscle). From there its proximal portion can often be seen as a white line in the subendocardium of the septomarginal trabeculation where it is still within a fibrous sheath. Distally, ramifications of the right bundle branch extend to the apex of the heart, and are also carried across the ventricular cavity through the moderator band and other muscular bundles (see Fig. 4.1). In some hearts, an additional bundle arises from the branching bundle, in between the bundle branches, and extends forward. This is described as the ‘dead end tract’ and is more often seen in fetal and infantile hearts than in adult hearts [17]. It continues from the main bundle antero-superiorly toward the root of the aorta.
Under the microscope, the specialised AV conduction bundle and its main branches are readily identifiable by their encasing fibrous sheaths using basic histological stains. In keeping with Tawara’s work [12], it is the continuity from section to section that serves as the most reliable method for histological localisation of the AV conduction system. Beginning with the AV node, which has the inherent function of delaying the cardiac impulse, the human node has a compact portion, and zones of transitional cells (see Fig. 4.4). The compact node is recognizable, when seen in cross-sections, as a half-moon shaped structure hugging the central fibrous body. The nodal cells are smaller than atrial myocytes. Like the cells of the sinus node, the compact nodal cells are closely grouped, and frequently arranged in interweaving fashion. In many hearts, the compact node has a stratified appearance with a deep layer overlain by a superficial layer. When traced inferiorly, toward the base of Koch’s triangle, the compact area separates into two prongs, usually with the artery supplying the node running in between. The prongs bifurcate toward the tricuspid and mitral annuli respectively. Their lengths vary from heart to heart and in recent years the rightward prongs have been implicated in so-called slow pathway conduction in AV nodal re-entrant tachycardia [18]. Interposing between the compact node and the working atrial myocardium is a zone of transitional cells (see Fig. 4.4b). These cells are histologically distinct from both the cells of the compact node and the working cells of the atrial myocardium, and are not insulated from the surrounding myocardium. The cells are long, attenuated, and have a wavy appearance. They tend to be separated from one another by thin fibrous strands. According to established definitions, transitional cells do not represent conducting tracts but they provide the crucial bridge between the working and the specialised myocardium. Transitional cells interpose between the left and right margins of the compact node and the myocardium from the left and right sides of the atrial septum. Wider extensions of transitional cells are present inferiorly and posteriorly between the compact node and the mouth of the coronary sinus and into the Eustachian ridge. The right margin of the node faces the vestibule of the right atrium. Here, an overlay of working myocytes in the subendocardium from the atrial wall in front of the fossa ovalis streams over the layer of transitional cells.
When the conduction system is followed distally from the compact node into the penetrating bundle of His, there can be little difference in the cellular composition in the two areas. The specialised cells themselves, however, become aligned in a more parallel fashion distally. Even so, Tawara [12] proposed that the distinction be made on purely anatomical grounds. The key change from node to bundle is that the bundle is insulated by fibrous tissue from the adjacent myocardium (see Fig. 4.6b), preventing atrial activity from bypassing the node. Thus, all atrial activity must be channeled via the AV node.
Being surrounded by fibrous tissue, the penetrating bundle is the first part of the axis which qualifies as a conducting tract (see Fig. 4.5b). The cells are marginally larger than compact nodal cells and they increase in size as the penetrating bundle continues into the AV bundle and branching bundle (Fig. 4.8). Here, the cells are very similar in size to ventricular myocytes. Swollen cells or Purkinje cells are not characteristic of specialised myocytes in the human heart and are seldom seen. However, they are typically seen in ungulates (Fig. 4.9) [12, 19, 20]. The AV bundle, branching bundle and proximal parts of the bundle branches are recognizable by the fibrous sheaths that encase them, insulating them from the adjacent working ventricular myocardium. When the bundles loose their fibrous sheaths distally, it is no longer possible to distinguish conduction tissues from working myocardium.
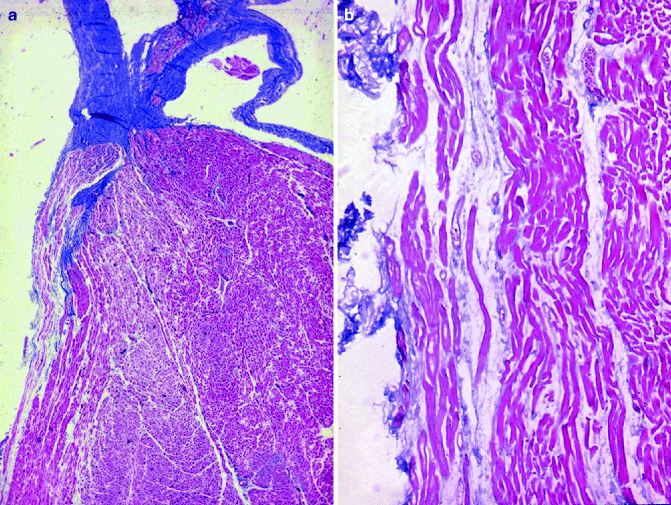
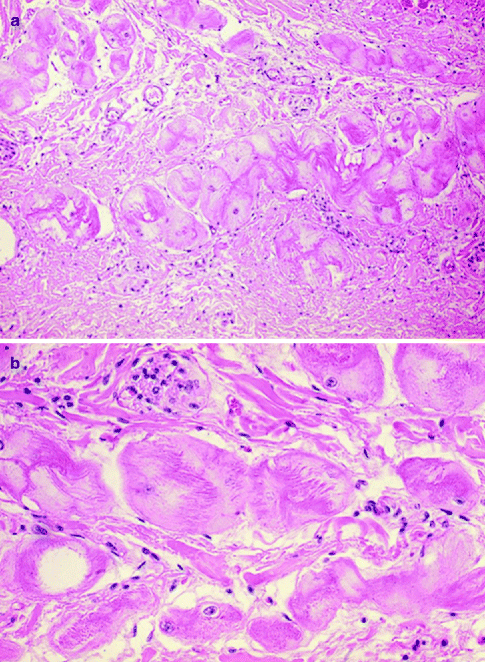

Figure 4–8.
(a) The course of the left bundle branch under the subendocardium of the left side of the ventricular septum (Heidenhain trichrome ×5); (b) Close-up of the Purkinje-like cells of the left bundle branch (Heidenhain trichrome ×25)

Figure 4–9.
Purkinje cells in ungulates’ heart. (a) Large, swollen and pale specialised cardiac myocytes as compared to the surrounding working myocardium (haematoxylin-eosin ×40); (b) Purkinje cells at higher magnification (haematoxylin-eosin ×120)
Pathology
Sino-Atrial Block and Sinus Arrest
The atrial activation may be impaired (atrial standstill) for two main reasons: impulses are not generated from the sinus node (sinus arrest) or their propagation to the atria is impeded (sinus block). In the etiology of sino-atrial block, several lesions of the sinus node and its innervation have been described, besides neurovegetative changes (vagal stimulation), drug sensitivity – intoxication and hyperkalemia.
From the pathological viewpoint, abnormalities of the sinus node artery, of the specialised myocardium of the sinus node and/or of its connections with the atrial myocardium (nodal approaches), and of the nodal ganglionated plexus have been reported. Myocardial infarction due to occlusion of the right coronary artery, proximal to the origin of the sinus node artery, remains the main cause of sinus node dysfunction, causing severe damage of the node and its atrial approaches in terms of necrosis, leukocytic infiltrates and haemorrhage. Sinus node artery perfusion can be also altered as a consequence of arteritis (Fig. 4.10) and embolism (Fig. 4.11), amyloid deposition and connective tissue disorders [21–25]. Recipient sinus node in cardiac transplantation undergoes to massive infarction due to nodal artery transection during surgical procedure and the donor sinus node artery may show obstructive intimal proliferations due to allograft vasculopathy (chronic rejection) (Fig. 4.12) [26].
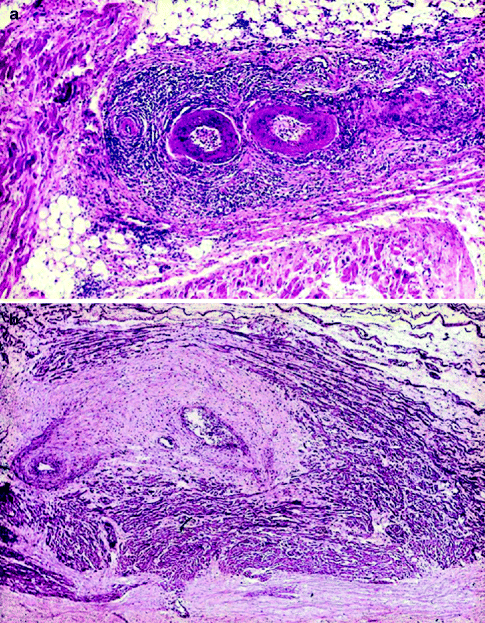
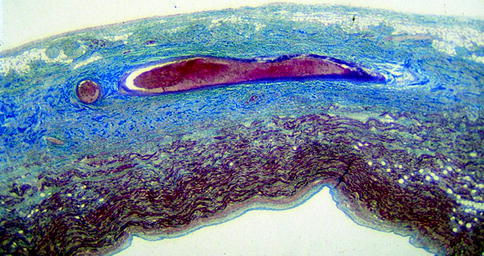
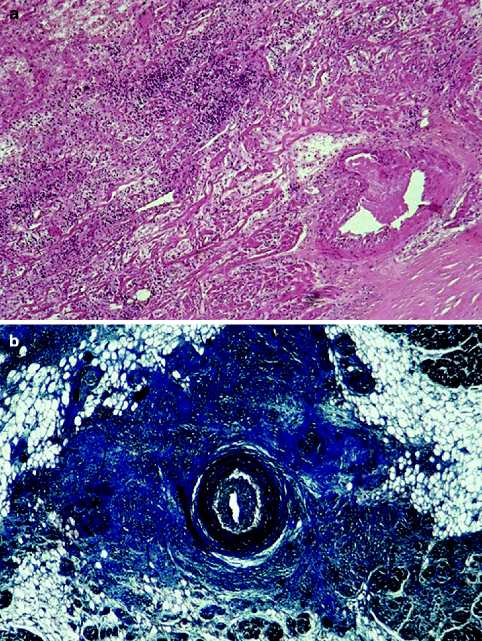

Figure 4–10.
(a) Arteritis of the sinus node artery in polyarteritis nodosa: note the inflammation extended to the nodal specialised myocardium (haematoxylin-eosin ×15); (b) AV node in polyarteritis nodosa: the AV nodal artery shows aneurysm and recanalized thrombus with extensive fibrotic replacement of conductive cells (haematoxylin-eosin ×15)

Figure 4–11.
Massive infarction of the sinus node and crista terminalis by occlusive thromboembolism of the nodal artery (Heidenhain trichrome ×6)

Figure 4–12.
Conduction system pathology in the transplanted heart. (a) AV node shows severe acute rejection and thrombosis of the AV node artery (haematoxylin-eosin stain, original magnification a ×60); (b) Histologic section of the donor sinus node artery in a patient with allograft vasculopathy (chronic rejection) who died 1,996 days after cardiac transplantation: note the obstructive, concentric intimal proliferation (Trichrome Heidenhain stain ×30)
AV Block
Any disease, either acute or chronic, that affects the myocardium may produce AV block, which may occur at the level of AV node approaches, AV node itself, penetrating or branching part of the bundle, and bundle branches [21–23, 27].
Morgagni, Adams and Stokes share the merit of having individuated the clinical entity of AV block and, after the recognition of the anatomic basis of the AV conduction axis by His and Tawara, Mahaim did the first clinico-pathologic assessment of this entity [28].
Pathologically, AV block may be classified as being caused by congenital or acquired diseases [27]. As far as the congenital AV block in an otherwise normally developed heart, this is usually a benign condition, mainly due to a lack of connection between the atria and the peripheral conduction system, with fatty replacement of the AV node and nodal approaches [29]. Moreover, the AV bundle may present marked fragmentation and septation. Maternal lupus with an immune mechanism plays a major etiopathogenetic role [30, 31].
Acquired AV block may be caused by acute myocardial ischemia or infarction. Inferior myocardial infarction may be complicated by third-degree AV block due to ischemic injury of the AV node itself. In particular, in postero-septal myocardial infarction, due to right coronary artery thrombotic occlusion in right dominant pattern, ischemic damage may involve atrial approaches to the AV node, AV node and His bundle. However, since the conducting tissues are resistant to ischemia, pathologic changes may be reversible and the AV block transient. Anterior myocardial infarction usually is associated with third-degree block due to ischemia or infarction of bundle branches; the branching bundle and bundle branches are often involved by the necrotic process, and by inflammatory infiltrates of the surrounding working ventricular myocardium [32]. Chronic ischemic heart disease, with or without infarction, may also be characterized by AV block, due to fibrotic changes of the bifurcating bundle and bundle branches, as well as of the crest of the ventricular septum [27].
The heart may be the target of angioitides and collagen diseases. Polyarteritis nodosa is a medium-large vessel vasculitis, with cardiac involvement in up to 80 % of cases. It typically manifests as pericarditis, coronary arteritis, myocardial infarction, arrhythmias and conduction disturbances [24]. Nodal arteries may be of the proper size to be affected and the surrounding conductive tissue involved as well (Fig. 4.10b).
Cardiac involvement has been reported in 8–44 % of cases of Wegener’s granulomatosis. It typically manifests as pericarditis, coronary arteritis and myocarditis with granulomas involving the conduction system.
Similarly, cardiac involvement is seen in up to 70 % of cases of systemic lupus erythematosus. The most common findings are pericarditis, myocarditis, and Libman-Sacks endocarditis. As previously mentioned, congenital heart block is a peculiar feature of neonatal lupus syndrome, which is associated with transplacental transfer of anti-Ro and anti-La antibodies [30, 31]. In rheumatoid arthritis, rheumatoid granulomas may affect the myocardium, endocardium, and valves as well as the conductive tissues; conduction disturbances and heart block have been reported [33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


