Detect Fluid Early from Intrathoracic Impedance Monitoring (DEFEAT-PE) is a prospective, multicenter study of multiple intrathoracic impedance vectors to detect pulmonary congestion (PC) events. Changes in intrathoracic impedance between the right ventricular (RV) coil and device can (RVcoil→Can) of implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy ICDs (CRT-Ds) are used clinically for the detection of PC events, but other impedance vectors and algorithms have not been studied prospectively. An initial 75-patient study was used to derive optimal impedance vectors to detect PC events, with 2 vector combinations selected for prospective analysis in DEFEAT-PE (ICD vectors: RVring→Can + RVcoil→Can, detection threshold 13 days; CRT-D vectors: left ventricular ring→Can + RVcoil→Can, detection threshold 14 days). Impedance changes were considered true positive if detected <30 days before an adjudicated PC event. One hundred sixty-two patients were enrolled (80 with ICDs and 82 with CRT-Ds), all with ≥1 previous PC event. One hundred forty-four patients provided study data, with 214 patient-years of follow-up and 139 PC events. Sensitivity for PC events of the prespecified algorithms was as follows: ICD: sensitivity 32.3%, false-positive rate 1.28 per patient-year; CRT-D: sensitivity 32.4%, false-positive rate 1.66 per patient-year. An alternative algorithm, ultimately approved by the US Food and Drug Administration (RVring→Can + RVcoil→Can, detection threshold 14 days), resulted in (for all patients) sensitivity of 21.6% and a false-positive rate of 0.9 per patient-year. The CRT-D thoracic impedance vector algorithm selected in the derivation study was not superior to the ICD algorithm RVring→Can + RVcoil→Can when studied prospectively. In conclusion, to achieve an acceptably low false-positive rate, the intrathoracic impedance algorithms studied in DEFEAT-PE resulted in low sensitivity for the prediction of heart failure events.
Highlights
- •
Prediction of CHF on the basis of thoracic impedance was examined.
- •
Impedance vectors from RV and LV leads were evaluated in ICD and CRT patients.
- •
Algorithms producing low false-positive rates had low sensitivity to detect CHF.
- •
Algorithms including LV and RV leads were not superior to RV lead–only algorithms.
- •
An algorithm involving the RV lead and can was FDA approved on the basis of this study.
Pulmonary congestion (PC) is an important determinant of symptoms and clinical outcomes in patients with congestive heart failure (CHF). PC can be detected by changes in intrathoracic impedance detected by implanted cardiac devices, traditionally with an impedance vector from the right ventricular (RV) coil to the device can (RVcoil→Can). Implantable cardioverter-defibrillators (ICDs), particularly dual-chamber ICDs and cardiac resynchronization therapy ICDs (CRT-Ds), have multiple lead electrodes, which may include right atrial, RV, and left ventricular (LV) electrodes, with multiple potential impedance vectors between these electrodes themselves and the device can in addition to the traditional RVcoil→Can vector. Incorporation of an impedance vector incorporating the LV lead (which may span and detect impedance changes over a different and potentially larger region of lung compared with RV-based impedance) was found to improve the detection of PC in preclinical large animal studies, compared with algorithms involving only the RV lead and can. Given the possibility that some of these impedance vectors (either alone or in combination) may be superior to the traditional RVcoil→Can vector in the detection of PC, a feasibility study was performed in 75 patients with CHF implanted with CRT-Ds (St. Jude Medical, Sylmar, California) to evaluate the relative performance of 6 different available vectors in the prediction of PC events. In this feasibility study, impedance vector combinations applicable to CRT-D patients (LVring→Can + RVcoil→Can, sensitivity 71.4%, false-positive rate [FPR] 0.56 per patient-year) and to all ICD patients (RVring→Can + RVcoil→Can, sensitivity 61.9%, FPR 0.63 per patient-year) resulted in optimal detection of PC events and were superior to the conventional, single-vector RVcoil→Can (sensitivity 57.1%, FPR 0.74 per patient-year). On the basis of the results of the feasibility study described here, Detect Fluid Early from Intrathoracic Impedance Monitoring (DEFEAT-PE) was designed as a multicenter clinical study to prospectively evaluate the performance of these vectors and vector combinations in detection of PC events.
Methods
This study was sponsored by St. Jude Medical and registered with ClinicalTrials.gov (identifier NCT00916929 ). Thirty-four centers participated in DEFEAT-PE. The patient population included in this study had either standard ICD or CRT-D indications on the basis of current guidelines. In addition, all patients were required to have diagnoses of heart failure substantiated by ≥1 documented episode of decompensated heart failure that required intravenous administration of diuretics or vasoactive drugs within the 6 months preceding enrollment. Patients were excluded for any of the following conditions: age <18 years, placement of an integrated bipolar ICD lead, presence of a capped or inactive right atrial or RV lead, placement of a non–St. Jude LV lead, placement of an epicardial lead, end-stage renal disease requiring dialysis, inotrope dependence or long-term home inotrope use, pregnancy or planned pregnancy within 6 months, current participation in a clinical trial with an active treatment arm, or life expectancy <6 months for any reason. All devices were required to have been implanted for ≥31 days before enrollment in the study to ensure that the leads were secured in place, the device pocket had stabilized, and the patient had sufficiently recovered from the device implantation. Signed informed consent was required before study enrollment.
St. Jude Medical ICD devices (models 1107-36, 1207-36, 1211-36/36Q, 2207-30/36, and 2211-36/36Q) and CRT-D devices (models 3107/3207-30/36, 3211-36Q, and 3215-36Q) with a diagnostic impedance monitoring feature were used in this study. Impedance monitoring is a device-based algorithm that measures intrathoracic impedance from up to 6 different vector configurations, including the pulse generator (can) and bipolar RA, RV, and LV leads. The devices used in the study have the capability to measure impedances in 6 independent vectors formed by the following electrode configurations: (1) LVring→RVring, (2) LVring→RAring, (3) RVring→Can, (4) LVring→Can, (5) RAring→Can, and (6) RVcoil→Can. On the basis of the results of the feasibility study, the impedance vectors preselected for prospective analysis in DEFEAT-PE were a combination of RVring→Can + RVcoil→Can for the ICD devices and a combination of LVring→Can + RVcoil→Can for the CRT-D devices ( Figure 1 ). Measurements from the ICD impedance vectors (combination of RVring→Can + RVcoil→Can) were also available for comparative analysis for the patients with CRT-D devices in DEFEAT-PE.
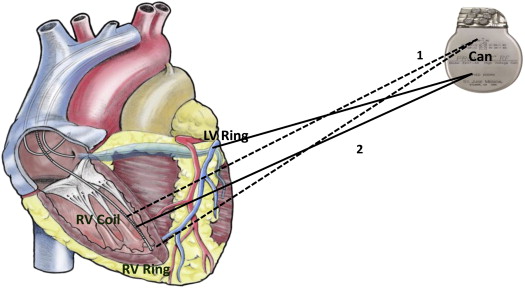
The impedance algorithm measures intrathoracic impedance every 2 hours from 2 vector configurations in the 2 device types (ICD and CRT-D). Impedance data from the 2 vectors are weighted and added together. The average values taken from the 2-hour samples are used to calculate the short-term average or daily impedance. Changes in short-term average or daily impedance over time determine a long-term average, or a reference impedance, which indicates the currently expected daily impedance value. If the daily impedance (short term) dips below the reference impedance (long term), this is termed a “sensed state of congestion.” If this sensed state of congestion is maintained for a specified duration (days), the device indicates that an algorithm-detected event has occurred. This first day of the algorithm-detected event is the first day that the sensed state of congestion has been maintained for the threshold number of days. The first day of the algorithm-detected event must occur <30 days before or during a clinical PC event to be considered true positive. This algorithm therefore detects congestion on the basis of the duration of deviation of the impedance from the reference value, rather than the amplitude of the impedance deviation from the reference. Further details of the impedance algorithm itself and of the classification of clinical events can be found in the published feasibility study.
After device implantation and study enrollment, patients had baseline visits 45 to 60 days after enrollment and then were seen at follow-up visits every 3 months for the duration of the study. Clinical events that occurred after the baseline visit were included in the primary and secondary end points. At each scheduled visit, device diagnostic information and impedance data were collected and downloaded to the St. Jude Medical Merlin Patient Care System. Clinical evaluations included New York Heart Association functional class and a medical interview to collect information on overall health since the previous scheduled visit. Cardiac devices were programmed at the physician’s discretion (with regard to both pacing and defibrillator therapies). All physicians and patients were blinded to the impedance data throughout the study. Enrollment was initiated in the study on June 9, 2009, with data collection completed on March 28, 2012.
All clinical PC events were adjudicated by a blinded 3-physician committee composed of heart failure specialists. This committee was given the task of incorporating all available clinical, laboratory, and x-ray data to determine whether each identified event was caused by PC. The adjudication committee physicians had full access to all available clinical data on each event, including descriptions of clinical findings, laboratory evaluation, x-rays, and procedural data but was blinded to the device impedance data. A clinical event was defined as any hospitalization or emergency room visit for PC or any urgent clinic visit requiring either intravenous diuretics or vasoactive drugs for PC. For patients who were seen in the emergency department or hospitalized, chest x-rays (minimum anteroposterior view), cardiac biomarker tests (N-terminal pro–B-type natriuretic peptide), and serum complete blood counts were collected at admission and before discharge. All chest x-rays were adjudicated by a blinded thoracic radiologist. Each chest x-ray was given a grading from 0 to 3 that classified the presence of PC. X-ray findings were used to grade each chest x-ray as 0 (normal), 1 (noncardiac PC), 2 (mild cardiac PC), or 3 (severe cardiac PC). The sample size calculation was based on the results of the previous feasibility study, with projected FPRs of 0.56 per patient-year (CRT-D algorithm) and 0.63 per patient-year (ICD algorithm), with the objective of demonstrating an FPR of <1.5 per patient-year. On this basis, the study was estimated to require 162 patients, with a total of 41.6 patient-years of follow-up for 80% study power with a 5% significance level.
Additional variables were also examined as potential predictors of PC events, including gender, the ejection fraction, age, device type, chronic obstructive pulmonary disease, cause of cardiomyopathy, history of myocardial infarction, percutaneous coronary intervention, unstable angina, and coronary artery bypass surgery. Univariate generalized linear regression models were used to determine the odds ratio (OR) and 95% confidence interval for each variable, and then the variables with significant (p <0.05) associations in the univariate models were entered into a multivariate regression model as covariates using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


