Heart failure with preserved left ventricular ejection fraction (HFpEF) is implicitly attributed to diastolic dysfunction, often recognized in elderly patients with hypertension, diabetes, and renal dysfunction. In these patients, left ventricular circumferential and longitudinal shortening is often impaired despite normal ejection fraction. The aim of this prospective study was to analyze circumferential and longitudinal shortening and their relations in patients with nonischemic HFpEF. Stress-corrected midwall shortening (sc-MS) and mitral annular peak systolic velocity (S′) were measured in 60 patients (mean age 73 ± 13 years) with chronic nonischemic HFpEF in stable New York Heart Association functional class II or III and compared to the values in 120 healthy controls and 120 patients with hypertension without HFpEF. Sc-MS was classified as low if <89% and S′ as low if <8.5 cm/s (the 10th-percentile values of healthy controls). Isolated low sc-MS was detected in 46% of patients with HFpEF, 27% of patients with hypertension, and 2% of controls; isolated low S′ was detected in 11% of patients with HFpEF, 7% of patients with hypertension, and 5% of controls; and combined low sc-MS and low S′ was detected in 26% of patients with HFpEF, 9% of patients with hypertension, and 5% of controls (HFpEF vs others, all p values <0.001). Thus, any alteration of systolic function was found in 83% of patients with HFpEF. The relation between sc-MS and S′ was nonlinear (cubic). Changes in S′ within normal values corresponded to negligible variations in sc-MS, whereas the progressive decrease below 8.5 cm/s was associated with substantial decrease in sc-MS. In conclusion, circumferential and/or longitudinal systolic dysfunction is present in most patients with HFpEF. Circumferential shortening normalized by wall stress identifies more patients with concealed left ventricular systolic dysfunction than longitudinal shortening.
Approximately half of patients with heart failure (HF) have preserved left ventricular (LV) ejection fraction. A number of studies have reported characteristics of cardiac geometry and function in patients classified as having HFpEF, but the results have not been consistent. A significant proportion of patients with HFpEF also have concomitant LV systolic dysfunction that is not detected by the simple measure of LV function at the chamber level. These patients may exhibit depressed longitudinal LV function, but also cross-sectional shortening at the midwall level may be reduced, mainly representing impairment of circumferentially oriented cardiomyocytes, possibly influenced in part by the function of longitudinally oriented myofibers. The relations between indexes of LV midwall and longitudinal function have never been investigated in these patients.
Accordingly, we evaluated the relations between circumferential midwall and longitudinal shortening in patients with HFpEF and evaluated the frequency of presentation of systolic dysfunction with either measure.
Methods
Patients participating in the Cardiac Geometry and Function in Heart Failure With Preserved Left Ventricular Ejection Fraction (CARRY-IN-HFpEF) study were 60 subjects with HFpEF aged >45 years in stable clinical conditions, sinus rhythm, and unchanged pharmacologic treatment during the 3 months preceding enrollment. They had histories of acute or worsening HF requiring hospitalization occurring 1 year to 3 months before enrollment. The diagnosis of HF at the time of the index hospital admission was based on a comprehensive assessment on the basis of modified Framingham criteria, clinical symptoms, response to diuretic and unloading therapy, and natriuretic peptides and was confirmed by an echocardiographic evaluation of diastolic function. Prerequisite criterion for eligibility was an echocardiographic LV ejection fraction ≥50% <24 hours after the HF event.
Exclusion criteria were a history of myocardial infarction or myocarditis, unstable clinical and/or hemodynamic conditions evaluated by echocardiography, myocardial ischemia (by electrocardiography and troponin plasma levels), or evidence of coronary artery disease diagnosed by clinical, electrocardiographic, and echocardiographic evaluation at rest and by the results of exercise scintigraphy or stress echocardiography or coronary angiography, primary hypertrophic cardiomyopathy, previous myocardial revascularization, and significant valvular disease. Patients were consecutively recruited from March 2008 to March 2010 at a single center. The study was conducted prospectively. Informed consent was obtained by all participants.
Patients with HFpEF were compared to 120 normotensive healthy volunteers without diabetes or any cardiovascular events. A group of 120 patients with hypertension without histories or symptoms and signs of HF was also studied. Patients with hypertension were receiving antihypertensive and, when needed, antidiabetic medications. Normotensive and hypertensive patients were enrolled prospectively during the recruitment period of HFpEF patients.
Concomitant hypertension was defined as high blood pressure (≥140/90 mm Hg) or the use of antihypertensive medications. Obesity was defined as a body mass index ≥30 kg/m 2 . Diabetes mellitus was diagnosed according to World Health Organization criteria (fasting serum glucose ≥126 mg/100 ml or 2-hour postchallenge serum glucose ≥200 mg/100 ml or use of hypoglycemic medication). Glomerular filtration rate estimated using the simplified Modification of Diet in Renal Disease (MDRD) equation was used for the assessment of renal function. Renal dysfunction was defined as a glomerular filtration rate <60 ml/min/1.73 m 2 .
Standard transthoracic Doppler echocardiographic studies were performed using a Megas Esaote Biomedica machine (Florence, Italy) equipped with a 2.5- to 3.5-MHz annular-array transducer. Echocardiographic measurements were blinded to the presence of HFpEF. LV chamber dimensions, wall thickness, and mass were measured according to the American Society of Echocardiography. LV mass was normalized for height 2.7 , and LV hypertrophy was defined as LV mass ≥49.2 g/m 2.7 for men and ≥46.7 g/m 2.7 for women. Relative wall thickness was calculated as 2 × the end-diastolic ratio of posterior wall thickness to LV diameter and indicated concentric LV geometry if ≥0.43 (the 97.5th percentile in the normal population). Excess LV mass relative to individual cardiac workload was assessed as the ratio between the observed value and the value predicted from stroke work, gender, and body size (as height 2.7 ) using a validated equation. For categorization, LV mass was defined as “inappropriate” when >128% of the predicted value (the 95th percentile of the normal adult distribution) and appropriate for values ≤128%. LV volumes were measured using the biplane method of disks and used to generate ejection fractions. LV systolic function was also assessed at the midwall level and related to end-systolic circumferential stress, as previously described. Stress-corrected midwall shortening (sc-MS) <89% (the 10th percentile of our healthy controls) indicated reduced LV contractility. Tissue Doppler imaging (spectral analysis) was used to measure peak mitral annular systolic velocity (peak S′, mean of 4 measurements detected in the septal, inferior, anterior, and posterior mitral annular position) as an estimate of longitudinal LV function. Peak S′ <8.5 cm/s (the 10th percentile of our healthy controls) indicated longitudinal systolic dysfunction. Mitral and pulmonary vein flow velocities and early diastolic tissue Doppler velocity (E′) were assessed according to the recommendations of the American Society of Echocardiography. Early diastolic velocity of transmitral flow (E) was divided by E′ and used to classify LV diastolic function together with other parameters (E/A ratio of transmitral flow, deceleration time of E, and the difference in duration of the atrial wave of pulmonary vein flow and the atrial wave of transmitral flow) in 4 degrees as proposed by Redfield et al : normal, mild dysfunction, moderate dysfunction, and severe dysfunction. Maximal left atrial volume was also computed from the 2-dimensional apical 4-chamber view using the area-length method and was normalized for height 3 .
Patients with HFpEF were statistically comparable to healthy controls and patients with hypertension for age, gender, body weight, and body mass index according to the following procedure: a Gower’s generalized distance from each of the healthy control was computed and ranked in ascending order. The distance was calculated using these variables ordered as follows: age, gender, body weight, and body mass index. The 120 healthy controls and 120 patients with hypertension were then defined by taking for each patient with HFpEF (n = 60) the 2 closest cases (selected from pools of 200 and 340 patients, respectively). Data are reported as mean ± SD. SPSS version 11.0 (SPSS, Inc., Chicago, Illinois) was used for statistical analysis. Unpaired Student’s t tests and chi-square statistics were used for descriptive statistics. Between-group comparisons of categorical and continuous variables were performed using chi-square tests and analysis of variance with post hoc comparison between each group using Scheffé’s test and Tukey’s honestly significantly different (Spjøtvoll-Stoline) test for unequal samples, as appropriate. Stepwise multiple linear regression analysis was used to identify independent correlates of LV circumferential or longitudinal indexes. In-model tolerance was used to evaluate multicollinearity. The minimal accepted tolerance was 0.80. Curve-fitting estimation analysis was performed to investigate the relations between the circumferential and longitudinal shortening of the myocardial fibers. The models considered for the estimation were linear, quadratic, cubic, compound, growth, logarithmic, exponential, inverse exponential, power, and logistic. To determine which model to use, we viewed the scatterplot of our data, analyzed the type of mathematical function, and transformed our data fitting to the corresponding type of available model. The r 2 coefficient was used as indicator of goodness of fit for each model. Two-tailed p values <0.05 were considered statistically significant.
Results
The study population consisted of 60 patients with HFpEF, whose main clinical and echocardiographic characteristics are compared to those of patients with hypertension and healthy controls in Table 1 and Table 2 , respectively. The 3 groups were by definition comparable for age, gender distribution, and body mass index.
| Variable | Patients With HFpEF (n = 60) | Patients With Hypertension (n = 120) | Healthy Controls (n = 120) |
|---|---|---|---|
| Clinical | |||
| Age (years) | 73 ± 13 | 72 ± 12 | 71 ± 11 |
| Women | 34 (57%) | 65 (54%) | 67 (56%) |
| Body mass index (kg/m 2 ) | 27.4 ± 6.0 | 27.5 ± 4.5 | 26.8 ± 4.0 |
| Obesity | 22 (37%) | 32 (27%) | 26 (22%) |
| Systolic blood pressure (mm Hg) | 139 ± 21 ⁎ | 140 ± 16 ⁎ | 133 ± 7 |
| Diastolic blood pressure (mm Hg) | 82 ± 8 † | 84 ± 6 | 81 ± 4 |
| Heart rate (beats/min) | 68 ± 10 | 68 ± 9 | 68 ± 10 |
| NYHA functional class | 2.8 ± 0.6 ⁎ , † | 1.0 ± 0 | 1.0 ± 0 |
| History of hypertension | 37 (62%) † | 120 (100%) | — |
| Diabetes mellitus | 7 (12%) | 14 (12%) | — |
| Chronic renal disease (GFR <60 ml/min/1.73) | 9 (15%) | 18 (15%) | — |
| Chronic obstructive pulmonary disease | 2 (3%) | 3 (2%) | — |
| Obstructive sleep apnea syndrome | 6 (10%) | 8 (7%) | — |
| Medications | |||
| β blockers | 29 (48%) | 48 (40%) | — |
| ACE inhibitors/ARBs | 34 (57%) † | 90 (75%) | — |
| Calcium antagonists | 12 (20%) | — | |
| Diuretics | 36 (60%) † | 32 (27%) | — |
| Statins | 6 (10%) | 18 (15%) | — |
| Antiplatelet agents | 12 (20%) | 30 (25%) | — |
| Variable | Patients With HFpEF (n = 60) | Patients With Hypertension (n = 120) | Healthy Controls (n = 120) |
|---|---|---|---|
| End-diastolic diameter (cm) | 5.0 ± 0.61 | 5.0 ± 0.5 | 4.8 ± 0.4 |
| Relative wall thickness | 0.45 ± 0.10 ⁎ , † | 0.40 ± 0.07 ⁎ | 0.37 ± 0.04 |
| LV mass index (g/m 2.7 ) | 58 ± 13 ⁎ , † | 48 ± 13 ⁎ | 37 ± 6 |
| LV hypertrophy | 46 (77%) ⁎ , † | 46 (38%) ⁎ | 4 (3%) |
| LV concentric geometry | 34 (56%) ⁎ , † | 40 (33%) ⁎ | 12 (10%) |
| Observed/measured LV mass (%) | 148 ± 45 ⁎ , † | 118 ± 30 ⁎ | 98 ± 16 |
| Inappropriately high LV mass | 37 (61%) | 34 (28%) | 5 (4%) |
| Maximal left atrial volume (ml/m 3 ) | 9.3 ± 3.2 ⁎ , † | 8.4 ± 2.8 ⁎ | 6.8 ± 2.2 |
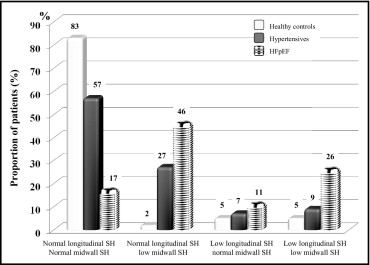
Figure 1 displays the prevalence of reduced midwall and/or longitudinal LV function in the 3 study groups. At least 1 parameter of LV systolic function was abnormal in 83% of patients with HFpEF, 43% of subjects with hypertension, and 12% of normal controls. Low sc-MS was more frequently detected than low peak S′ ( Table 3 ). Midwall and longitudinal dysfunction coexisted in 26% of patients with HFpEF.
| Variable | Patients With HFpEF (n = 60) | Patients With Hypertension (n = 120) | Healthy Controls (n = 120) |
|---|---|---|---|
| Midwall shortening (%) | 14.7 ± 3.0 ⁎ , † | 17.3 ± 2.9 | 19.2 ± 2.4 |
| sc-MS (%) | 78 ± 16 ⁎ , † | 92 ± 16 ⁎ | 102 ± 12 |
| Low sc-MS | 43 (72%) ⁎ , † | 43 (36%) ⁎ | 8 (7%) |
| LV ejection fraction (%) | 60 ± 6 ⁎ | 60 ± 10 ⁎ | 64 ± 6 |
| Peak S′ velocity (cm/s) | 9.5 ± 3.0 ⁎ | 10.5 ± 2.3 | 10.8 ± 3.0 |
| Low S′ velocity (<8.5 cm/s) | 22 (37%) ⁎ , † | 19 (16%) ⁎ | 12 (10%) |
| Peak E′ velocity (cm/s) | 8.8 ± 2.5 ⁎ , † | 9.6 ± 2.3 | 10.1 ± 2.3 |
| Mitral E/A ratio (cm/s) | 0.93 ± 0.49 | 0.85 ± 0.28 | 0.89 ± 0.27 |
| E/E′ ratio | 7.7 ± 3.2 ⁎ , † | 6.5 ± 2.0 | 6.0 ± 1.6 |
| Diastolic dysfunction | 47 (80%) ⁎ , † | 72 (60%) ⁎ | 54 (45%) |
| Grade I | 34 (57%) | 61 (51%) | 54 (45%) |
| Grade II | 8 (13%) | 11 (9%) | 0 |
| Grade III | 6 (10%) | 0 | 0 |
A first logistic regression analysis was performed to compare the parameters of LV systolic and diastolic function in patients with HFpEF to those in patients with hypertension and controls. The statistical model indicated that sc-MS (exponent β = 0.95, 95% confidence interval 0.93 to 0.97, p = 0.001) was independently associated with the condition of HFpEF, while peak S′ (exponent β = 0.97, 95% confidence interval 0.78 to 1.20, p = 0.77) and E/E′ ratio (exponent β = 1.16, 95% confidence interval 0.99 to 1.36, p = 0.07) were not.
The relation between sc-MS and peak S′ was tested using multiple linear regression analysis. Two statistical models were performed, the first including all patients with HFpEF, patients with hypertension, and healthy controls and the second focused on the 60 patients with HFpEF. In the 2 models, sc-MS was considered the dependent variable; the following confounding variables were added to peak S′ in the list of potential covariates: age, gender, body mass index, heart rate, history of hypertension, diabetes, LV mass, appropriateness of LV mass, E/A ratio, E/E′ ratio, deceleration time, maximal left atrial volume, relative wall thickness, antihypertensive drugs, and HFpEF (only in the first model). The analysis proved the positive association between indexes of LV circumferential and longitudinal function, independent of the presence of higher relative wall thickness, inappropriate LV mass, and lower body mass index, all conditions related to lower sc-MS. In the first model (model A), HFpEF also emerged as a condition related to lower sc-MS ( Table 4 ). Tolerance was optimal, demonstrating the loss of significant collinearity among the variables in the 2 statistical models.



