Blood pressure measure
Systolic
Diastolic
R-value
SDD
CV
R-value
SDD
CV
Office
0.48
17.0
11.0
0.31
10.0
10.0
24-h mean
0.87a
9.8a
7.0
0.90a
4.7a
5.6
Awake
0.86a
10.7a
7.4
0.88a
5.8a
6.5
Sleep
0.92a
7.7
6.3
0.88a
5.2
7.1
In contrast, there may be little advantage of ambulatory BP over clinic pressure in clinical trials when the measure of interest is a small block of time, such as 1 or 2 h. In an evaluation of a group of hypertensives using both noninvasive and intra-arterial BP over 24 h, Mancia et al. [23] showed that the standard deviation of the differences for 24-h mean BP values was similar for the two methodologies. However, despite the much larger number of BP values obtained in an intra-arterial study, the hourly BP reproducibility was no different for the direct measurements than it was for noninvasive BP monitoring. Thus, unlike the larger blocks of time (e.g., 24 h, the awake period, or the sleep period), the reproducibility of hourly ambulatory BP data has not been shown to be superior to that of office BP measurements [23]. Hence, ambulatory BP recordings will not allow a reduction in sample sizes if the end point is for short periods of time. This increased variability in hourly BP levels is probably secondary to differences in the activities of patients monitored on two different days. Because controlling for hourly physical and mental activities is nearly impossible in free-ranging subjects, longer periods of time (e.g., 4 h) will remain statistically and practically appropriate for clinical trials involving ambulatory BP monitoring.
The benefits of ambulatory monitoring of the BP over clinic BP with regard to sample size requirements in clinical trials of the elderly may be substantially reduced, however. One report in elderly hypertensive patients with isolated systolic hypertension suggests that there would not be improvement in reproducibility for ambulatory BP over clinic BP measurements, as the between-subject variance was not much different for the two methods [24]. Staessen et al. reported that 60 subjects with isolated systolic hypertension would be required to detect a 10-mmHg difference in systolic BP between two treatments in a parallel design using clinic readings, whereas if ambulatory BP monitoring was used, the number would only fall to 54. In contrast, if a crossover design was used, the number of subjects needed to show the same systolic BP difference would be 18 and 14, respectively, for clinic vs ambulatory BP measurements.
Ambulatory Blood Pressure for Evaluating Patients for Clinical Trial Entry
At the recruitment and enrollment phases of a clinical hypertension trial, current antihypertensive medication is discontinued, and baseline BPs and heart rates are obtained during a single-blind placebo period that generally lasts for 2–4 weeks. Conventional inclusion criteria for these trials have been based on the seated clinic systolic or diastolic BPs at the end of the single-blind placebo period. In recent years, many protocols have also included ambulatory BP values as primary or secondary criteria for inclusion into the study. For example, it is not uncommon to require that the seated diastolic BP in the clinic exceed 90 or 95 mmHg and that the awake (or daytime) ambulatory BP exceed 85 or 90 mmHg [1]. The impact of various awake ambulatory BP values for exclusion of patients during the screening process can be fairly dramatic when the requirement for the office diastolic BP is 90 mmHg or greater.
An example of this is shown in an analysis from a multicenter US clinical trial (Fig. 20.1). In this study, a seated clinic diastolic BP of >95 and <115 mmHg was used as the criterion for entering and remaining in the single-blind portion of the trial. To progress into the double-blind randomized portion of the study, ambulatory BP values at certain thresholds were used. However, when using an ambulatory awake BP cutoff value of 85 mmHg, nearly 30 % of the study group was excluded from randomization into the double-blind part of the trial.
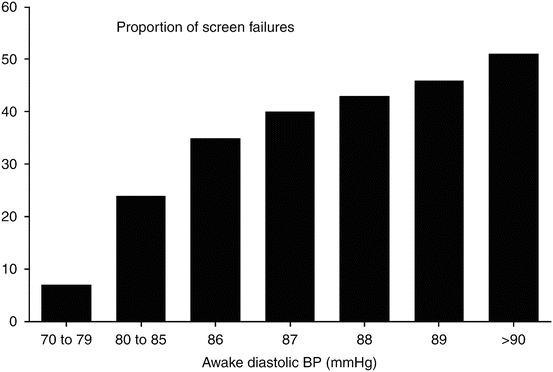

Fig. 20.1
Effects of patient recruitment using an office diastolic blood pressure >90 mmHg and various awake ambulatory diastolic blood pressures as the selection criteria in antihypertensive drug trials (Modified from [1])
When 90 mmHg was the cutoff value for ambulatory diastolic pressure, more than 50 % of the group was excluded from randomization. The major reason for the high exclusion rate based on the ambulatory BP values compared to the office pressure is that a relatively high percentage of patients entering these trials experience a white coat effect [3, 7, 25, 26]. There has always been substantial controversy as to whether patients with white coat hypertension should be included or excluded from participation in clinical trials of antihypertensive drugs. The viewpoint of those favoring inclusion of white coat hypertensive patients is that it is relevant to study their hemodynamic response to the new drug because it is likely that many patients with the white coat syndrome will have therapy prescribed by physicians who base treatment decisions solely on office BP measurements. Although not an unreasonable concern, a number of studies have demonstrated that patients with white coat hypertension exhibit few changes in ambulatory BP following antihypertensive therapy [27, 28]. The typical compromise in recent years has been to include an intermediate cutoff BP entry value for ambulatory BP that is lower than the office BP entry criteria. By using the ambulatory BP criteria, fewer patients will be required in the randomized portion of the study, but the number of individuals screened in the single-blind period is typically increased [9, 20, 21]. Even in 2015, it remains commonplace to recruit about 130–140 % of the required final number of patients who can be evaluated to assure that the statistical power required to show the desired changes from baseline will be achieved.
Multicenter Trials to Evaluate Antihypertensive Therapy
A multicenter trial is a clinical trial conducted simultaneously by several investigators working in different institutions, but using the same protocol and identical methods in order to pool the data collected and analyze them together [29, 30]. Two key reasons for conducting multicenter studies rather than single-center studies is to enhance patient recruitment and to make the study group more “representative” of the entire patient population, as there may be unique or homogeneous local population characteristics from a single center.
Analyses of Multicenter Hypertension Trials: Relevant Problems for Ambulatory Blood Pressure Recordings
There are several problems specific to multicenter hypertension trials , including those utilizing ambulatory BP measurements. First, there is the “center” factor: incorporation of the center factor into the analysis improves the power of comparison of treatments. One can assess the variability among centers from the residual variability in order to improve the sensitivity in detecting differences between treatments. However, in the extreme situation of 1–2 patients in a center, it is not possible to differentiate between a center-related effect and one related to the treatments. In such cases, the smallest centers should be pooled to serve as a larger “center” in the statistical model.
Second, it is important to evaluate whether the differences between treatment modalities vary outside of random fluctuations across all centers. If a major difference does exist, it may be proven statistically by performing a test of treatment by center interaction.
It is relevant to evaluate the causes of treatment by center interactions because they may invalidate the principal findings of a study [31], including those using ambulatory BP monitoring. Some of the causes of treatment-by-center interactions are listed in Table 20.2. An abnormal or unusual frequency of protocol violations, losses to follow-up, or noncompliant patients may occur in one or more centers. When this occurs, it may reflect poor adherence to the protocol or insufficient motivation of the investigators or study coordinators at these centers. For example, when a center has inexperienced or apathetic personnel involved in an ambulatory BP monitoring protocol, there may be excessive data loss during the recording period, poor correlation between cuff and device measurements, or inaccuracy with regard to drug dosing and recorder hookup times [3, 7, 32].
Table 20.2
Causes of treatment-by-center interactions in multicenter clinical trials
Differences in patient features from one center to another |
Abnormal frequency of protocol violations, losses to follow-up, or noncompliance in one or more centers |
Outlier center (small number of “doubtful” cases) |
Real variations of differences between treatments according to centers |
Outlier centers, possibly because of a small number of doubtful cases, may decrease credibility concerning these data and may also cause a center effect. However, tests for outliers may be misleading in a large clinical trial. For example, if three or four centers are identified as outliers, it is possible that these are the clinics that are doing a good job while the others are not. Thus, statistical tests for outliers, which are essentially tests of homogeneity, must be used carefully and intelligently by the statisticians in charge of the final analyses. Finally, real variations of differences between treatments according to centers may occur and restrict the general applicability of the results.
Bias in a Multicenter Trial Using Ambulatory Blood Pressure Monitoring
Bias enters into a multicenter trial through two primary mechanisms: selection bias and misclassification bias. An example of selection bias in an antihypertensive drug trial involving ambulatory BP monitoring might be recruiting a more severely hypertensive population to avoid inclusion of white coat hypertensives. Many investigators have learned to avoid screen failures (Fig. 20.1), which has a possible financial impact on the center, so they will enroll either a patient with higher clinic pressures or one who has a “known” ambulatory BP from a previous trial. Misclassification bias in the multicenter trial is exemplified by clinic BP measurement error (e.g., using improper methodology for measurement) and may induce a center effect if untrained or inexperienced personnel are used. Misclassification bias is probably uncommon in studies using experienced investigators and coordinators, but in recent multinational, multicenter trials, the use of centers inexperienced in performing clinical research and measurement errors have become more prevalent.
Practical Concerns in the Conduct of Multicenter Trials That Use Ambulatory Blood Pressure Recorders
The use of ambulatory BP monitors in a clinical trial requires the availability of skilled, trained technicians. Multiple devices are necessary in order to complete a clinical trial in a timely fashion, to facilitate the scheduling of large numbers of participants and to minimize the impact of mechanical problems when they occur. These requirements increase study costs, but are critical to the success of the trial. An additional concern is the possibility that the data for some individuals may be excluded from analyses because of the poor quality of an ambulatory BP recording or mechanical difficulty [33]. Rarely, the use of ambulatory BP monitoring may hinder recruitment efforts because certain individuals may decline participation in a trial as a result of the perceived burden of wearing and returning a recorder. Appel et al. [33] have reported that patient recruitment efforts improve when the technique is presented as a standard part of the study. Then the expectation is that the ambulatory BP data collection is a primary part of the study [34] as opposed to an optional or ancillary procedure.
Importance of the Placebo Effect in Clinic vs. Ambulatory Blood Pressure
It remains standard practice to include a placebo group in clinical trials involving antihypertensive therapy, especially studies that are considered dose ranging or “pivotal” during the earlier phases of drug development. Because of the marked variability of office BP, most investigators have found it necessary to distinguish true drug effect from placebo effect in BP trials. Several factors might create a reduction in BP on placebo, including regression to the mean [34], the presence of a pressor response in the doctor’s office that resolves the white coat effect [35, 36], and expectations of the patient and the clinical observer [5]. Unfortunately, surreptitious readings may also play a role in BP reductions on placebo.
In contrast to studies that employ clinic pressure as the primary means to obtain the primary study end point, the placebo effect is either minimal or absent when ambulatory BP monitoring is used in antihypertensive drug trials [37–39]. The lack of observed placebo effect on ambulatory BP is probably secondary to both the lack of observer bias and the increased number of BP values obtained over a 24-h study period. In contrast, ambulatory BP monitoring probably would not remove regression to the mean or other potential patient factors that contribute to the placebo effect. In fact, it is somewhat difficult to separate the regression to the mean effect from the placebo effect and the decrease in a white coat effect over time. This phenomenon of regression to the mean in an ambulatory BP monitoring study has been shown quite clearly in a study by White et al. [40]. In this trial, patients with hypertension were randomized to receive either placebo or controlled-onset extended-release (COER) verapamil for 8 weeks after a 3–4-week single-blind placebo baseline period. The patients were then divided into those with BP fall by >10 % during sleep compared to their awake values (dippers) and those with BP falls by <10 % during sleep (non-dippers). In the non-dipper patients randomized to receive placebo, nocturnal systolic BP fell by 4 mmHg compared to the first baseline study (also on placebo but during the single-blind period). In the dipper patients randomized to receive placebo, nocturnal systolic BP increased by about 4 mmHg compared to the baseline BP. Thus, the spread between the two types of patients in the placebo group for nocturnal pressure was nearly 8 mmHg (Fig. 20.2).
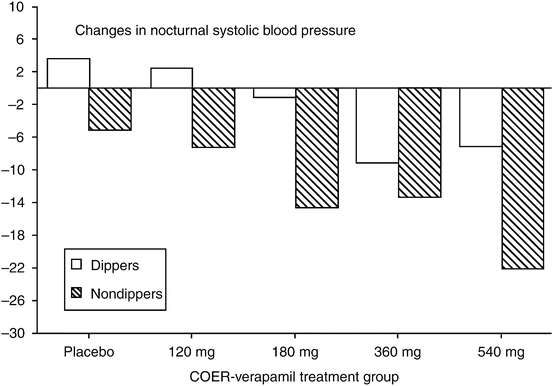

Fig. 20.2
Changes in nocturnal (10 PM to 5 AM) blood pressure (BP) from baseline measured by ambulatory BP monitoring in dippers (decline in mean BP from the daytime period to the nocturnal period was >10 %) contrasted with changes in nocturnal BP in non-dippers (<10 % decline in nocturnal BP) on placebo and four doses of controlled-onset extended-release (COER) verapamil (Modified from [40])
The changes in nocturnal BP on active drug were also consistently greater across all doses and greater in non-dippers compared to dippers. Thus, if this substantial regression to the mean on placebo had not been accounted for, the response to active treatment with COER verapamil in the dippers would have been underestimated and the response in the non-dippers would have been overestimated.
More recently, Bakris et al. [41] randomized 849 patients with resistant hypertension to darusentan (a selective endothelin-A receptor antagonist), placebo, and guanfacine (central α-2 agonist). At 14 weeks, the systolic BP in the placebo group decreased by 14 ± 14 mmHg in the office measurements, while the mean 24-h systolic BP only decreased by 2 ± 12 mmHg. Hence, when mean 24-h BP is taken into account, the placebo effect is dramatically attenuated. While the drop in clinic systolic BP at 14 weeks with darusentan was not significantly different from placebo, the mean 24-h systolic BP was lower for darusentan group (p < 0.001) (Fig. 20.3).
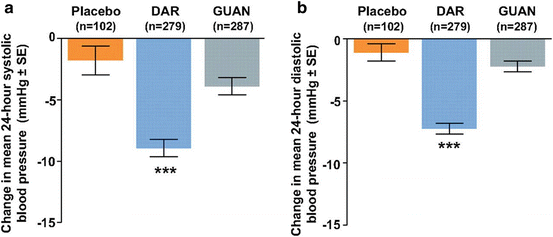

Fig. 20.3
Difference in mean 24-h ambulatory systolic (a) and diastolic (b) BP after 14 weeks of treatment with placebo, darusentan, and guanfacine. ***p < 0.001 as compared with placebo or guanfacine (From [41])
A study by Mutti et al. [39] reported a significant 10/3 ± 3/2 mmHg fall in office BP after 4 weeks of placebo, whereas the overall 24-h BP was unchanged. However, the ambulatory BP did fall slightly during the first 8 h of placebo administration, which the authors attributed to the white coat effect. Of note is that this initial fall did not have a statistical effect on the overall mean BP and thus ambulatory BP monitoring still allowed for better characterization of the placebo effect.
In a much longer-term study by Staessen et al. [42] in older patients with isolated systolic hypertension, one treatment arm was randomized to placebo for 1 year. Compared to the baseline period, the clinic systolic BP fell by 7 ± 16 mmHg on placebo and the 24-h systolic BP fell by just 2 ± 11 mmHg. Because the ambulatory BP has a superior repeatability index compared to the clinic pressure, the changes in 24-h systolic BP met statistical significance, whereas the larger mean reduction in clinic BP showed only a trend (p = 0.06). Because this was a patient population with normal diastolic BP, there were no statistically significant changes in clinic or ambulatory diastolic BP on placebo therapy during this study. The authors also noted that the 24-h systolic BP was more likely to decrease on placebo if it was higher at baseline and if the follow-up was longer. The authors discounted regression to the mean or the white coat effect as having an impact on these placebo effects and recommended that long-term studies in older patients using noninvasive ambulatory BP monitoring require a placebo-controlled design.
Analysis of Ambulatory Blood Pressure Data in Antihypertensive Drug Trials
Data from ambulatory BP studies in hypertension trials can be analyzed in a number of ways (Table 20.3). Features of any method of analysis for ambulatory BP data should include the statistical ease of calculation, clinical relevance of the measure, and relationship of the parameter to the hypertensive disease process. The most popular methods remain the change from baseline in the 24-h systolic and diastolic BP and the visual assessment of the hourly curves over 24 h. However, the use of daytime and nighttime means (or preferably awake and sleep values), blood pressure loads (the proportion of values above a cutoff value during wakefulness [>140/90 mmHg] or sleep [>120/80 mmHg] divided by the total number of BP readings), area under the 24-h BP curve (AUC), and smoothing techniques designed to remove some of the variability from the raw BP data analysis are also among the utilized methods of analysis [1–4, 9, 10].
Table 20.3
Methods for evaluating ambulatory blood pressure data in clinical trials
24-h means (and standard deviation as a measure of variability) |
Awake and sleep means based on patient diaries or activity recorders |
Hourly means |
Blood pressure load (proportion method) |
Area under the blood pressure curve |
Placebo-subtracted curves showing hourly means |
Various data smoothing techniques (including cosinor analyses or fast Fourier transformation) |
Modeling of 24-h curves |
Trough-to-peak ratio (for once or twice daily drugs) |
The 24-h mean BP remains an important parameter for evaluation in antihypertensive drug trials because it appears to be a strong predictor of hypertensive target organ disease [2], is easy to calculate, utilizes all of the ambulatory BP data, and as previously mentioned, is remarkably reproducible in both short-term [6] and long-term [21] studies.
The blood pressure load has been used as a simple method of analysis in evaluating the effects of antihypertensive drugs. Blood pressure load has been defined as the percentage of BPs exceeding 140/90 mmHg while the patient is awake plus the percentage of BPs exceeding 120/80 mmHg during sleep [43]. A number of years ago, we evaluated the relationship between this BP load and cardiac target organ indexes in previously untreated hypertensives. At a 40 % diastolic BP load, the incidence of LVH was nearly 80 %, but below a 40 % diastolic BP load, the prevalence of LVH fell to about 8 %. In contrast, the office BP and even the 24-h average BP were not as discriminating in predicting LVH in this group of previously untreated patients. Thus, in mild to moderately hypertensive patients, one would desire a low (conservatively <30 %) BP load while being treated with antihypertensive drug therapy.
In studies in which the patient population has a greater range in BP, the proportional (or percentage) BP load becomes less useful. Because the upper limit of the BP load is 100 %, this value may represent a substantial number of individuals with broad ranges of moderate to severe hypertension. To overcome this problem, we devised a method to integrate the area under the ambulatory BP curve and relate its values to predicting hemodynamic indices in untreated essential hypertensives [10]. Areas under the BP curve were computed separately for periods of wakefulness and sleep and combined to form the 24-h AUC. Threshold values were used to calculate AUC such as 135 or 140 mmHg systolic while awake and 85 or 90 mmHg diastolic while awake. Values during sleep were reduced to 115 and 120 mmHg systolic and 75 and 80 mmHg diastolic.
Smoothing of ambulatory BP data may be used to aid in the identification of the peak and trough effects of an antihypertensive drug. The extent of variability in an individual’s BP curve may be large as a result of both mental and physical activity; thus, evaluating the peak antihypertensive effect of a short- or intermediate-acting drug may be difficult. Other than the benefits associated with examining pharmacodynamic effects of new antihypertensive drugs, data and curve smoothing for 24-h BP monitoring appear to have little clinical relevance. Furthermore, editing protocols are not uniform in the literature, and missing data may alter the balance of mean values for shorter periods of time. To avoid excessive data reduction in a clinical trial, one statistical expert suggested that data smoothing should be performed on individual BP profiles rather than on group means [44].
Situations in Which Ambulatory Blood Pressure Monitoring Has Been Useful in Antihypertensive Drug Trials
Treating the White-Coat Hypertensive Patient
The inclusion of white coat hypertensive patients in an antihypertensive drug trial that uses only clinic BP criteria for study entry will have a potentially confounding effect on efficacy, because these patients are not hypertensive outside of the medical care environment [7, 8, 11, 13]. Furthermore, patients may develop excessive drug-induced side effects without much change in BP, especially if titration of the dose is based on office pressures.
In a study by Weber et al. [28], a sustained fall in BP was found across a study group taking a long-acting form of diltiazem. In a subset of six patients who had hypertensive office BP readings but whose ambulatory BPs were normotensive (i.e., a white coat hypertensive group), no significant ambulatory BP changes from placebo baseline (0/1 mmHg) were observed. In contrast, the diltiazem therapy decreased 24-h BPs by 18/13 mmHg in the subgroup of nine patients who were hypertensive by both office and ambulatory BP. Thus, treating white coat hypertensive patients may be of little to no benefit if the only place where BP reduction is observed is in the medical care environment.
Utility of Ambulatory Blood Pressure Monitoring in Dose-Finding Studies
Since the early 1990s, numerous studies have been performed with ambulatory BP monitoring to fully assess the efficacy of a wide range of doses of new antihypertensive agents. The advantage of ambulatory BP monitoring in dose-finding studies is the ability to discern differences among the doses with smaller numbers of study participants compared to the sample size requirements when clinic pressures are used.
Examples are shown next.
Eplerenone
The efficacy of eplerenone, a novel selective aldosterone receptor antagonist, was studied in patients with essential hypertension using a multicenter, randomized, placebo-controlled design [45]. In this trial, the drug was assessed using a once-daily dosing regimen of either 25 or 200 mg. Clinic and ambulatory BPs were compared to both baseline values and the effects of placebo. As shown in Fig. 20.4, there was a dose-related reduction in BP at the trough for both clinic and ambulatory BP. The ambulatory BP values were better at delineating the dose–response than the clinic BP readings.
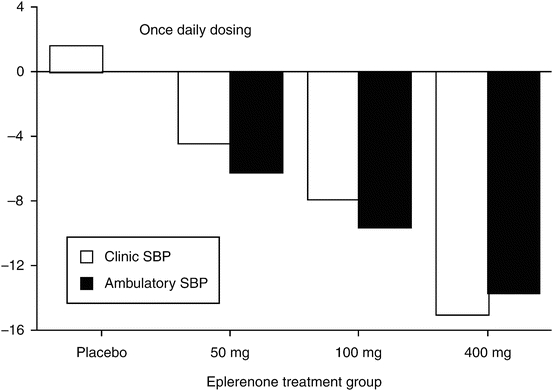





Fig. 20.4
Changes from baseline in ambulatory vs clinic systolic blood pressure in a dose-ranging trial with a selective aldosterone antagonist, eplerenone (Data from [45])
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


