Fig. 14.1
Prevalence of dipping patterns according to stage of chronic kidney disease. Classifications defined in terms of the sleep-time relative SBP decline: ≥20 % (extreme-dipper), 10–20 % (dipper), 0–10 % (non-dipper), <0 % (riser). From Hermida et al. [11], with permission
Evaluation of Adverse Clinical Outcomes in CKD 1–4
Hypertension is a major risk factor for the progression of CKD of any etiology and is a major risk factor for cardiovascular events and death. Because out-of-office BP is a better predictor of target organ damage than office BP and diurnal BP changes may add to this prediction, it was hypothesized that ABPM could be a better predictor of these major adverse events in CKD. This has been evaluated using both overall measures of 24-h BP control and circadian BP patterns.
In a prospective study on the course of events linking BP and albuminuria in type I diabetes, 75 young patients were followed for an average of 63 months and underwent ABPM every two years [12]. There was an increase in nighttime BP in the group who developed microalbuminuria, and this increase in BP preceded the identification of albumin in the urine, raising a possible pathophysiologic link between increase nighttime BP and albuminuria. In a logistic regression model, a 5 mmHg rise in nighttime systolic BP resulted in a 44 % increased risk of development of albuminuria on subsequent examinations. Office BP was unable to detect these differences, and daytime BP was less prominently changed [12]. These findings pointed to the importance of the pattern of nocturnal BP to the progression of renal abnormalities. Likewise, several small studies suggested that the rate of loss of renal function and/or increase in proteinuria was greater in non-dipping than dipping patients with different causes of CKD [13], diabetic nephropathy [14], and IgA nephropathy [15]. However, more recent, larger studies have failed to corroborate the independent importance of non-dipping in predicting CKD progression after adjustments for average BP and other factors [16, 17], as discussed in detail below.
Target-Organ Dysfunction, Morbidity, and Mortality in CKD 1–4
As in essential HTN, ambulatory BP is better than office BP to predict left ventricular mass index in nondiabetic patients with CKD [18]. In hypertensive patients with IgA nephropathy, left ventricular mass was significantly related to nighttime blood pressure and “diurnal index” (% BP decline at night), but there was no relationship with daytime BP [19]. In normotensive patients with autosomal dominant polycystic kidney disease with normal renal function, left ventricular mass was higher as compared to health control subjects. The increase in left ventricular mass index was related to ambulatory BP. The nocturnal decrease in BP was also attenuated in polycystic kidney disease patients, but it was not associated with the increased left ventricular mass [20].
In a cross-sectional analysis of a cohort of 617 African-American patients enrolled in AASK study, ABPM identified patients with more extensive target-organ damage based on nighttime BP levels despite similar clinic and daytime BP [9]. When subjects were divided according to tertiles of nighttime SBP, those in the highest tertile (nighttime SBP >142 mmHg) had significantly lower GFR (41 vs. 44 mL/min/1.73 m2 compared with lowest tertile), more proteinuria (0.55 vs. 0.17 g/g creatinine), and higher prevalence of LVH on echocardiography (81 % vs. 57 %) [9]. Such differences would not have been captured by clinic or daytime BP, thus making it also unlikely that home BP would have been distinctive. Another cross-sectional study of 232 male patients with CKD has corroborated the stronger association between ABPM with proteinuria than a standardized office BP measurement [21].
There has been progress in identifying the prognostic role of ABPM and home BP monitoring in patients with CKD. Agarwal and Andersen enrolled 217 male patients with CKD due to multiple etiologies, mostly diabetes and hypertension (baseline eGFR 45 mL/min/1.73 m2), to evaluate the development of ESRD or death according to standardized office BP or home BP [22]. After a median follow-up of 3.5 years, one standard deviation of home systolic BP (21 mmHg) was associated with 84 % increased risk (95 % CI = 1.46–2.32) of death or progression to ESRD after adjustments for demographic factors, office BP, and baseline proteinuria and eGFR [22]. The point estimate of decreased risk for ESRD alone was similar (74 %, 95 % CI = 1.04–2.93). In a companion paper published shortly thereafter, the same authors presented ABPM data for the same cohort showing that one standard deviation increase in 24-h systolic BP (17 mmHg) resulted in a 62 % (95 % CI—1.21–2.18) increase in risk of ESRD or death after adjustment for clinic BP [16]. However, this prognostic advantage did not remain significant after adjustment for other clinical factors (including age, diabetic status, and baseline proteinuria and eGFR). Of the individual components of ABPM, only nighttime systolic BP was a significant predictor of death and ESRD risk after multiple adjustments (hazard ratio 1.79 for ESRD or death, 1.90 for death) [16].
In a multicenter study, Minutolo et al. enlisted 436 patients with CKD of varying etiologies, mostly hypertension, diabetes, and tubulointerstitial diseases (baseline eGFR 43 mL/min/1.73 m2) [23]. After 4.2 years of follow-up, only ABPM values, not office, were associated with cardiovascular events, progression to ESRD, or death. As noted in Fig. 14.2, there was a progressive increase in the risk of cardiorenal outcomes with increasing levels of baseline daytime and nighttime ambulatory BP, but not office BP. This group has recently published further data on outcomes based on the degree of BP control in the office, on ABPM, both or neither [24]. They considered office BP as being at goal is less than 140/90 mmHg, whereas ABPM was considered at goal if daytime BP was <135/85 mmHg and nighttime BP was <120/70 mmHg. Among the 489 study subjects, 17 % were controlled both at home and office, 22 % were controlled only on ABPM (i.e., a white coat effect), 15 % only in the office (i.e., a masked effect), and 47 % on neither (“uncontrolled”). The group with a “white coat effect” had similar risk of cardiovascular events, dialysis, and death as the referent group (controlled BP in both settings). Conversely, the “masked effect” and uncontrolled groups had 2.3–3.9-fold greater risk of all adverse outcomes than patients with controlled BP on adjusted analyses [24]. The authors also performed several sensitivity analyses using different BP cutoffs that provided relatively similar results.
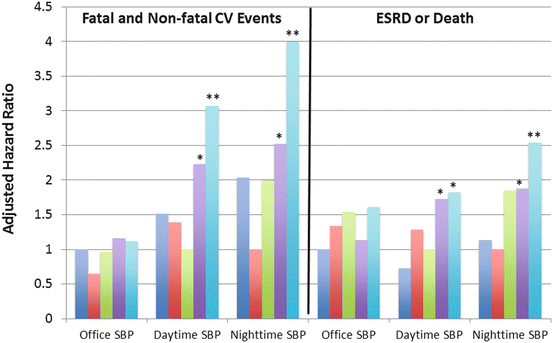

Fig. 14.2
Risk of cardiovascular and renal events in patients with chronic kidney disease according to office or ambulatory BP measurements. CV cardiovascular, ESRD end-stage renal disease, SBP systolic blood pressure. BP categories for each type of measurement (based on quintiles of the distribution): Office SBP (mmHg): <130 (reference), 130–139, 140–149, 150–159, >159. Daytime SBP (mmHg): <118, 118–125, 126–135 (reference), 136–146, >146. Nighttime SBP (mmHg): <106, 106–114 (reference), 115–124, 125–137, >137. Hazard ratios adjusted for age, gender, body mass index, diabetes, cardiovascular disease, hemoglobin, proteinuria, and baseline renal function. *p < 0.05, **p < 0.01. Data compiled from Minutolo et al. [23]
Finally, the 5-year longitudinal analysis of 617 patients with hypertensive nephrosclerosis enrolled in the AASK study found ABPM to be better than office BP for prediction of loss of renal function and cardiovascular events [17]. Both daytime and nighttime BP remained significant predictors of cardiovascular events despite adjustments for office BP and other covariates. On the other hand, ABPM values were only associated with the composite renal endpoint (doubling of serum creatinine, ESRD or death) in patients with controlled office BP (systolic BP <130 mmHg). The investigators postulated that because they used standardized office BP measurements, this may have decreased the observed differences between ABPM and office BP, and that these differences were only relevant in patients with a possible masked effect (i.e., those with controlled office BP). As previously mentioned, dipping status was not an independent predictor on any outcomes in AASK [17].
In summary, similar to the general population, out-of-office BP measurements, both ABPM and home BP, are better predictors of renal and cardiovascular outcomes in patients with CKD. The studies, however, are not as well-powered as studies in other hypertensive populations, and there are inconsistencies regarding the prognostic value of individual ambulatory BP variables (i.e., daytime vs. nighttime vs. 24-h average vs. dipping status).
ABPM in Hemodialysis and Peritoneal Dialysis
Blood Pressure Profile in Dialysis Patients
Hemodialysis (HD) provides a unique scenario of BP variability making ABPM a potentially valuable diagnostic tool. Fluid gain between HD sessions, represented by the increase in body weight, is removed by ultrafiltration. This reduction in extracellular volume is usually accompanied by a fall in BP [25, 26]. The characteristic intermittence of HD treatments and wide fluctuations of extracellular volume during and between HD sessions produce a unique BP profile in HD patients. In conventional, thrice weekly HD, 44-h interdialytic ABPM shows that both awake and sleep BP increase between HD sessions [27, 28] (Fig. 14.3), and that this is not influenced by HD shift assignment (morning or afternoon) [28]. Although interdialytic fluid retention plays a major role in BP increase [29], several studies failed to find a correlation between interdialytic weight gain and interdialytic BP [28, 30]. Most of the ABPM descriptive studies showed that non-dipping pattern is very common in HD patients; more than two thirds of patients lack the normal diurnal BP rhythm [7]. Limited ABPM data are available in patients receiving more frequent (daily HD) or more prolonged (nocturnal HD) regimens that usually are associated with a better clinic BP control [31].
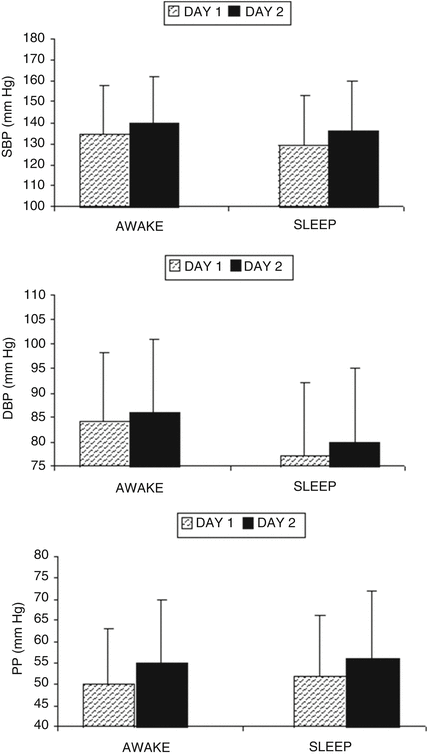

Fig. 14.3
Ambulatory blood pressure values on each interdialytic day during 44-h interdialytic monitoring in hemodialysis patients. SBP systolic BP, DBP diastolic BP, PP pulse pressure, *p < 0.05. From: Santos, F.F. et al. (2003) Am. J. Nephrol. 23, 96-105, with permission from S. Karger AG, Basel
ABPM has been used to determine reasonable clinic BP references in HD patients, i.e., those BPs taken before (pre-HD BP) and after (post-HD BP) HD sessions that best predict interdialytic BP [32–35]. In a systematic review and meta-analysis which included 18 studies where in-center BP and interdialytic ABPM were available, Agarwal et al. showed that pre-dialysis BP overestimated interdialytic ABPM, while on the other hand post-dialysis underestimated ABPM [33]. Although this meta-analysis raises significant concerns about the use of peri-dialysis BP levels in clinical practice, these are still the only measurements available to most physicians caring for HD patients. Recognizing this, we believe that pre-, post- and intradialytic dialysis BP can be used in a qualitative manner to diagnose interdialytic HTN. Agarwal and Lewis [33] have published clinic BP thresholds that have an accuracy rate of about 80 % to predict a diagnosis of HTN on ABPM (“hypertension” defined as 44-h interdialytic BP >135/85 mmHg). The best sensitivity and specificity are observed using pre-HD BP averages between 140 and 150/80 and 85 mmHg and post-HD BP averages between 130 and 140/70 and 80 mmHg [33]. Data from our group [32] and others [34] indicate that the best estimate of interdialytic BP lies around the average of pre-HD and post-HD BP levels. Indeed, Agarwal et al. [36] validated different indices of peri and intra-dialysis BP measurements and showed that a median of all intradialytic BP measurements including pre and post-dialysis BP was better reproducible and had the best accuracy and precision and the least bias compared to interdialytic ABPM. They also proposed a median intradialytic SBP of ≥140 mmHg (80 % sensitivity and 80 % specificity) and a median intradialytic DBP of ≥80 mmHg (75 % sensitivity and 75 % specificity) as thresholds for diagnosing interdialytic HTN.
Out-of-office BP (ABPM and home BP) is a useful tool to evaluate the efficacy of HTN treatment and to explore the association between BP and interdialytic symptoms . ABPM has been used to monitor dosing periods of antihypertensive drugs and home BP is superior to clinic BP for the titration of antihypertensive therapy in HD patients [37–39]. Moreover, because HD patients often have abnormal intradialytic BP changes, both hypo and hypertension [40], their BP control may be better assessed by interdialytic BP [41]. In an important paper, Battle et al. showed that a substantial portion of HD patients have a delayed BP nadir occurring 4–6 h following HD [41]. Van Buren et al. assessed the interdialytic BP profile of patients with intradialytic HTN (systolic BP increase >10 mmHg from pre- to post-hemodialysis in at least four of six treatments) and compared it with control individuals who had an intradialytic BP fall. Patients with intradialytic HTN had higher mean ambulatory SBP (both daytime and nighttime). Patients with intradialytic HTN had a slight decrease in SBP in the first nocturnal period, while BP increased in the control group. Thereafter, BP increased in both groups [42]. Agarwal and Light used ABPM to evaluate the role of volume overload in intradialytic HTN. They analyzed the intradialytic BP slope at baseline and after probing dry weight [43]. Patients who lost more weight were characterized by an intradialytic rise in BP at baseline. After effective lowering of dry weight, the slope changed to a net BP fall during HD. This observation suggests that lack of BP decline during HD is a sign of covert volume overload.
Decisions about dialysis prescription and timing of antihypertensive drug administration can thereby be made more effectively after understanding the duration of BP control (or even low BP) after each dialysis session. Home BP monitoring is a valuable adjunct in this assessment [44], and we often use it in our practice.
Fluid removal in peritoneal dialysis methods is continuous, and patients do not have large fluctuations in BP related to extracellular volume changes. Therefore, standard methods can be used to assess BP in PD subjects, who are usually seen monthly in the ambulatory setting. ABPM descriptive studies showed a blunted decline in nighttime BP in peritoneal dialysis patients that is very similar to the profile of HD patients [45, 46]. Different PD modalities (conventional ambulatory, cycler assisted) seem to have the same diurnal BP profiles [45, 47]. Differences in BP profile according to peritoneal transport characteristics were explored [48]. While there was substantial difference in the prevalence of HTN and average BP levels (elevated in high and high-average transporters), there were no differences in the diurnal BP profile among groups.
ABPM and Home BP and Target-Organ Dysfunction, Morbidity, and Mortality in Dialysis Patients
The relationship between HTN and outcomes in dialysis patients is complex. While high BP is associated with adverse outcomes, low BP has an even stronger association with mortality , and the optimal levels of BP control are still uncertain for patients on HD or peritoneal dialysis [2]. Despite this uncertainty, HTN remains a central concern in the care of dialysis patients, and ambulatory BP measurements have added substantially to the understanding of the relationships between BP and outcomes in these patients.
Several cross-sectional studies have studied the relationships between office BP, ambulatory BP, and LVH in dialysis patients. Two studies, both in patients undergoing long, slow HD, showed no significant correlation between BP levels, office or ambulatory, and LVH [49, 50]. Others have shown higher correlation coefficients between ambulatory BP and LVH than those linking office BP and LVH in conventional HD patients [26, 27, 51–54], though others have not confirmed this observation [55]. Understanding these differences is not straightforward. Study design, echocardiographic methods, and ABPM techniques were relatively similar among studies. It is possible that the use of a composite of many peri-dialysis readings (or home BP readings for that matter) improves the consistency of the results in such a way that the correlations between LVH and office BPs become indistinguishable from the more consistent and reproducible ABPM values [55, 56]. Furthermore, because dipping is blunted, average casual and ambulatory BPs tend to be more similar, and differences in prediction may be minimized. One last possible factor is that echocardiographic determination of left ventricular mass (LVM) depends on assumptions of cardiac symmetry that are not always present in HD and is dependent on the state of hydration of the patient, which inevitably fluctuates in HD subjects. Echocardiograms underestimate LVM at low LVM values and overestimate it at higher LVM values, a bias that is amplified in patients with higher end-diastolic volumes (a possible indicator of latent volume overload) [57].
Another question that has been addressed by some investigators is whether the circadian BP rhythm matters in the evaluation of LVH in dialysis. Amar et al. did not observe any relationship between dipping status and echocardiographic LVH [58], whereas other investigators have found that the degree of dipping correlates with LVM on univariate, but not multivariate analysis [50], and that nocturnal systolic BP is the strongest predictor of posterior LV wall thickness, though not of other indices of LVH [52]. Wang et al. showed that nocturnal systolic BP load >30 % (% of readings above 125/80 during the night) was the only significant predictor of LVH in a group of peritoneal dialysis patients [47]. In the only available longitudinal study in dialysis, Covic et al. followed echocardiographic and ABPM changes over 12 months and established that patients with a blunted circadian BP rhythm had progressive LV dilatation but no other markers of LV dysfunction on follow-up [59]. Overall, it remains uncertain whether ABPM adds to the evaluation of LVH or if these observations bear any prognostic value to dialysis patients.
Arterial stiffness is a strong predictor of mortality in ESRD [60]. The relationship between ABPM and pulse wave velocity (a marker of arterial stiffness) was studied by Amar et al. in 42 hemodialysis patients [58]. Casual and ambulatory BP had similar correlations with pulse wave velocity. However, non-dipper patients had significantly higher pulse wave velocity than patients with preserved diurnal rhythm (14.1 m/s vs. 11.5 m/s, p = 0.03). Karpetas et al. [61] performed concomitant 48-h ABPM and pulse wave velocity measurements in a cohort of HD patients. They observed a small increase in pulse wave velocity in the second interdialytic day. Older age and higher ambulatory mean BP were the factors independently associated with higher pulse wave velocity [61]. Agarwal and Light [29] found that arterial stiffness was associated with an overall increase in BP during the interdialytic period, while interdialytic weight gain, which is a predictor of mortality in HD, was associated with interdialytic increase in BP.
ABPM has been evaluated as a predictor of cardiovascular events among dialysis patients [62–65]. In one of the larger studies, Tripepi et al. followed 168 nondiabetic hemodialysis patients who had not sustained a previous cardiovascular event, 49 of whom died during an average follow-up of 38 months. Patients in the highest tertile of the night/day ratio (>1.01, i.e., reverse dippers) had an adjusted hazard ratio of 2.5 for total mortality and 4.3 for cardiovascular mortality [64]. Of relevance, blood pressure (either daytime or nighttime) was not associated with increased mortality risk. In the largest available study to date, Agarwal investigated a prospective cohort of 326 HD patients followed for up to 6 years (median 29 months). Dialysis unit BP (average of 2 weeks), home BP (thrice daily for 1 week), and 44-h ABPM were analyzed as predictors of all-cause mortality. ABPM and home blood pressure were similar predictors of mortality (adjusted hazard ratios for increasing quartiles of ABPM: 2.51, 3.43, 2.62; home BP: 2.15, 1.7, 1.44), and both were better than dialysis unit BP (likelihood ratio test p = 0.009). Only SBP was predictive of mortality and mortality was lowest when home SBP was between 120 and 130 mmHg and ABPM was between 110 and 120 mmHg [65].
In summary, ABPM and home BP are better predictors of adverse outcomes in dialysis patients as compared to clinic BP.
Technical Issues Specific to Dialysis Patients
Dialysis patients , and more specifically those on hemodialysis , have several features that may make BP measurement and ABPM difficult. These include the presence of arteriovenous grafts or fistulas, which alter blood flow in the extremities, the frequent changes in volume status represented by each dialysis session, and the BP fluctuation in BP throughout the period of 48–72 h separating one dialysis session and the next. Because of these shortcomings, particular concern exists to have devices formally validated in these patients.
In one relevant study, Fagugli et al. monitored 44 patients during the interdialytic period with an ABPM device that is capable of simultaneous oscillometric and auscultatory measurements (A&D Takeda Tm2421) to evaluate the relative accuracy of each technique in hemodialysis patients [66]. The oscillometric component of the device performed better based on several measures: standard deviations of all BP’s were lower (18.7 mmHg vs. 20.4 mmHg for systolic, 10.9 mmHg vs. 12.6 mmHg for diastolic, both p < 0.01); coefficients of variation were also lower (14.6 % vs. 16.1 % for systolic, 14.6 % vs. 17.7 % for diastolic, both p < 0.01); and percentages of valid BP readings were higher (94 % vs. 72 %, p = 0.001). The authors concluded that the oscillometric method was preferable in this group of patients. No other study has compared these two methods, but we have validated an oscillometric device in hemodialysis patients (SpaceLabs 90207) with acceptable results [67].
The effects of arteriovenous fistulas or grafts have not been systematically assessed. Nonetheless, in a validation protocol, we demonstrated that an oscillometric device performed equally well in patients with an arteriovenous graft/fistula as in patients with intact arms undergoing hemodialysis via a tunneled venous catheter [67].
Fluctuations in volume status raise issues about the reproducibility of ABPM in hemodialysis patients. To address this question, we evaluated the reproducibility of ABPM compared with dialysis clinic BP in 21 hemodialysis patients evaluated on average 68 days apart [68]. This study confirmed the wide BP variability in these patients. However, despite this shortcoming, ABPM had lower coefficients of variation and tighter limits of agreement than isolated clinic readings or a composite of readings from the week surrounding the monitoring period. We concluded that ABPM provides more reproducible BP values in hemodialysis patients. When unavailable, a composite of clinic readings are a suitable alternative (investigators have used averages of 1–4 weeks), whereas isolated readings should not be used to make any management decisions [69].
A last but important issue is the need to evaluate the entire interdialytic period and attendant BP fluctuations. This need is compromised by the fact that, in our experience, up to a third of patients are unable to complete a full 2-day monitoring period. As mentioned before, home BP monitoring is a very informative and inexpensive technique, which has the ability to provide comparable information on BP profile and prognosis.
ABPM in Adult Kidney Transplant Recipients
Cardiovascular disease is the leading cause of morbidity and mortality following successful kidney transplantation [70]. Furthermore, cardiovascular disease is a significant cause of death with a functioning allograft [70–74]. Estimates indicate that fatal and nonfatal cardiovascular events are approximately 50-fold more likely in transplant recipients compared with the general population [75]. As poorly controlled hypertension is a well-established risk factor for cardiovascular disease, optimal blood pressure management in the kidney transplant recipient is crucial. Confirming a beneficial effect from management, hypertension control is associated with prolonged allograft survival, even after censoring allograft loss for patient death [76, 77]. As hypertension is a modifiable risk factor, optimal control of blood pressure is essential following kidney transplantation.
Hypertension is present in the majority of kidney transplant recipients [78]. A recent study found only 16 % of recipients to be normotensive without the need for antihypertensive therapy [79]. Consistent with the general population, this study also showed insufficient control of hypertension in 44 %, while 10 % had WCH and 18 % had masked hypertension. Notably, only 16 % of the recipients studied had a normal nocturnal dipping blood pressure pattern. This increased incidence of hypertension is in part a consequence of the immunosuppression regimen. In particular, corticosteroids and the calcineurin inhibitors (cyclosporine more so than tacrolimus) are associated with hypertension [80]. Furthermore, and consistent with native kidney disease, hypertension can be both a cause and a consequence of allograft renal insufficiency.
The RETENAL Study compared assessments of blood pressure control in a cohort of 868 kidney transplant recipients in Spain by ABPM and clinic-based blood pressure measurement [78]. The study found 34 % of participants to have controlled ambulatory blood pressure. Circadian BP patterns showed a high proportion of risers (48 %) in addition to 34 % non-dippers, and only a small proportion (14 %) were dippers [78].
Azancot et al. recently studied the effect of transplantation on hypertension using office BP and ABPM [81]. They hypothesized that the immunosuppressants would lead to a greater degree of hypertension compared to patients with CKD and similar levels of renal insufficiency. The study design required both groups of patients to have moderate impairment in kidney function defined as an eGFR less than 60 mL/min. The office-based blood pressure assessments were comparable between the two groups. Using ABPM, however, a significant difference in both awake and asleep blood pressures was found between the groups, and transplant recipients were less likely to exhibit a normal diurnal blood pressure rhythm (21 % were dippers, compared with 34 % of the patients with CKD) [81]. In summary, nocturnal hypertension and non-dipping, both higher cardiovascular risk blood pressure patterns, were more significant and prevalent in transplant recipients, respectively.
Home Blood Pressure Monitoring (HBPM ) has also been investigated following kidney transplantation. Consistent with data from the general population [82], HBPM in kidney transplant recipients more closely correlated with ABPM data than did office-based recordings (72 % concordance vs. 54 %) [83]. Moreover, compared with ABPM reference data, HBPM was both more sensitive and specific at detecting hypertension than office-based BP measurements for the recipients studied.
Limited data are available to compare prognostic accuracy of office and ambulatory BP in renal transplant recipients. As in other groups of patients, ABPM is a better correlate of left ventricular hypertrophy than office BP in renal transplant patients [84, 85]. In a small prospective study of 46 renal transplant recipients undergoing ABPM at 6 and 18 months post-transplantation, ABPM values, but not office BP, were positively correlated with serum creatinine [86]. In a study of 119 transplant recipients, Wadei et al. used ABPM to assess loss of GFR and histological evidence of vascular injury in the allograft 12 months post-transplantation [87]. The analysis focused on circadian BP variability and they did not report on differences in outcomes according to office or average ABPM. As it pertains to circadian variability, they observed that the absence of nocturnal SBP reduction was strongly associated with lower GFR and greater vascular injury in the allograft [87], although this was not observed by another group of investigators using serum creatinine as the outcome variable [88].
The only long-term study evaluating graft failure and cardiovascular events in renal transplant patients included 126 patients followed for 46 months [89]. All patients underwent ABPM at 3 months post-transplantation. In this small study, the presence of a reverse dipper pattern on ABPM was associated with a 3.6-fold increase in risk of loss of allograft or cardiovascular event during follow-up (p = 0.02). Neither office BP nor other measures derived from ABPM were associated with the outcomes in question [89].
ABPM in Donor Nephrectomy Candidates
At the present time, there are over 100,000 registrants on the kidney transplant waiting list in the United States. This number significantly eclipses the number of deceased donor transplants performed annually, resulting in a significant donor organ shortage. Programs have adapted to this organ shortage through improved utilization of non-standard criteria donor organs, use of organs from deceased donors with cardiac death, and increased efforts to encourage recipients to seek a living donor. Indeed, most programs have a growing experience with allowing donor candidates with well-controlled hypertension to proceed with donor nephrectomy. In addition, there has been a significant upward shift in the average age of kidney transplant recipients, and with it an increase in the rate of transplantation from spouse to spouse. As a consequence, more donor candidates in middle and later life are presenting for evaluation. Many will already have a history of hypertension or prehypertension . Even more will have an elevated office blood pressure measured during their donor exam, raising concern for undiagnosed hypertension vs. white coat effect.
Multiple studies indicate that donor candidates with hypertension are at risk for worsened BP control following kidney donation [90–92]. Furthermore, one study correlated the degree of renal function decline associated with kidney donation with the donor’s BP prior to the nephrectomy, showing a greater decline in the donors with the higher preoperative BP measurements [92]. Given these findings and concerns, most transplant centers as part of their evaluation will obtain an ABPM study on donor candidates with high blood pressure detected in the office. Ommen et al. reported their experience using ABPM to determine the rates of hypertension, masked hypertension, and white coat hypertension in their single center cohort of potential donors [93]. A diagnosis of WCH was made in 62 % of the donor candidates and masked HTN in 17 %. By screening all of their donor candidates with ABPM, the author’s concluded that they had more accurately assessed the medical risk of the donor candidate while at the same time reducing their disqualification rate.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


