Chapter 10
Alternative Routes of Catheter Placement
Patients who require chronic long-term central venous access are at risk for occlusion of the central access veins. The risk of catheter-related central venous thrombosis is related to the patient’s underlying disease, the access site, and the device characteristics. The rate of catheter-related central venous thrombosis is therefore difficult to determine but approaches 30% in some populations.1 As more access sites become occluded, the provision of a secure, functional long-term central venous access becomes challenging. This chapter reviews the techniques that have been devised for insertion of central venous access devices in the patient with limited access options.
PATIENT ASSESSMENT
Patient assessment should begin with a clinical history, physical examination, and review of prior imaging studies and procedural records. Some patients may present with a history of multiple prior central venous catheters, failed attempts at central venous access, infusion of sclerosing medications through peripheral intravenous lines, intravenous drug abuse, dialysis, plasmapheresis, or surgical interruption of the central veins. Specific questions should be asked regarding past episodes of extremity swelling or known hypercoagulable conditions. Additional risk factors include mediastinal masses or adenopathy, fibrosing mediastinitis, and mediastinal radiation therapy. The number and location of previous central venous catheters should be determined. In addition, the presence and location of devices such as pacemakers, venous stents, and vena cava filters are important when planning venous access procedure.2
On physical examination, a swollen extremity with prominent superficial veins may be noted (Table 10–1). Dilated veins should be traced from their origin to their termination. Upper-extremity veins that are confluent with abdominal wall veins are highly suggestive of superior vena cava (SVC) occlusion. Scars from prior central venous catheters, dialysis access, or other surgical procedures are important clues to the patient’s condition. The extent of a radiation portal sometimes can be estimated from the small permanent tattoos applied by therapists to guide therapy or from changes in skin appearance.
The amount of information available from prior imaging studies is always surprising. The extent and age of an occlusion sometimes can be determined. Important anatomic variants or pathology in adjacent structures may be found when old studies are reviewed prior to a venous access procedure.
When prior imaging studies are not available, a thorough evaluation of all possible alternative venous access routes is essential. This evaluation should include both the peripheral and central veins. In particular, the status of the SVC and inferior vena cava (IVC) is critical information because these are the target vessels for most alternative access strategies. Cross-sectional vascular imaging modalities as well as conventional venography may be required.
JOHN A. KAUFMAN
Table 10–1 Patient Assessment
History | Findings at Physical Examination |
|---|---|
Prior catheters | Scars from prior accesses |
Prior DVT | Edema, dilated superficial veins, cords |
Dialysis accesses, shunts/fistulas | Scars, functioning or old |
Radiation | Simulation tattoos, erythema |
Hypercoagulable conditions | Adenopathy, surgical scars, cords |
DVT, deep venous thrombosis.
Ultrasound (US) can determine the patency of the veins of the upper and lower extremity and the deep veins of the neck. Gray-scale compression US can provide definitive information regarding the presence of venous thrombosis.3,4 The addition of Doppler waveform analysis and color-flow imaging improves the identification of patent venous segments. Unfortunately, because of the thickness of the overlying structures,4 this modality is of limited utility for assessing the central veins of the chest and abdomen. Patency of the central veins can be inferred from gray-scale or Doppler waveforms in the peripheral vessels, but cross-sectional imaging with computed tomography (CT) or magnetic resonance (MR) is preferable.
Both CT and MR can provide useful information when evaluating a patient for central venous occlusion or planning an alternative access.5,6 Contrast enhancement is essential for venous imaging with CT, with attention to the route of access (i.e., the nonsymptomatic side) and acquisition of a delayed scan to visualize veins. Venous studies with MR can be performed without contrast, although gadolinium-enhanced acquisitions are optimal. Both these modalities produce data that can be manipulated with image postprocessing tools to depict the venous structures better. A major advantage of both CT and MR is the ability to image clearly the deep central veins of the chest and abdomen. In addition, the anatomy of adjacent structures and the presence of pathology can be evaluated.
Conventional venography remains essential in the evaluation of patients with limited venous access. This simple and safe procedure provides an enormous amount of information regarding the nature and extent of the venous occlusions. Perhaps most important, collateral pathways are preferentially filled, showing the site of reconstitution and allowing the operator to select targets for central venous access. Bilateral upper-extremity venograms should be performed when evaluating the central veins. The jugular veins are not normally opacified during upper-extremity injections, an important limitation of this technique; however, jugular veins can be evaluated with ultrasound in conjunction with the upper-extremity venograms.
PREVENTION OF CATHETER-RELATED CENTRAL VENOUS THROMBOSIS
The best strategy for patients with limited central venous access is prevention of the problem in the first place. Therefore, reasonable attempts should be made to minimize venous trauma during insertion of catheters. The smallest diameter catheter for a particular need should be inserted, and a central location of the catheter tip should be ensured (Fig. 10–1). Administration of 1 mg of oral warfarin (Coumadin) daily significantly decreases catheter-related central venous thrombosis in oncology patients.1 Administration of low-molecular-weight heparin, 2500 IU subcutaneously daily, may prove equally beneficial.7 Catheters coated with heparin or other medications, which may render catheters less thrombogenic, are not yet available.
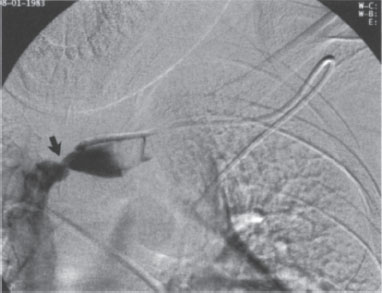
Figure 10–1 Injection through a left subclavian Hickman catheter. The catheter tip has withdrawn into the proximal left brachiocephalic vein. A stenosis (arrow) is present distal to the catheter tip, and a fibrin cap is present on the catheter itself. The catheter, in place for about 1 year, has malfunctioned for several months.
TECHNIQUES FOR ALTERNATIVE CENTRAL VENOUS ACCESS
Alternative access procedures should be performed with the same attention to detail as conventional access procedures. Patient monitoring, conscious sedation, surgical scrub technique, and prophylactic antibiotics are used. Usually, alternative access procedures are longer and more complicated and difficult than standard catheter placements, and so a conscious effort to maintain sterile technique is necessary.
Recanalization
Inserting a long-term central venous access catheter through a vein that is already occluded is the ideal procedure in patients with limited access for the simple reason that no new veins are placed at risk and are saved for future use.8 At our institution, this is the preferred technique for patients with limited access. The objective of this approach is only to place a catheter, not to recanalize the vein in the usual sense of relieving an obstruction, but both goals can be accomplished simultaneously if desired.9 This approach uses familiar access routes and recanalization tools (Fig. 10–2). There is a risk, however, that this approach will fail after expenditure of much time and effort, requiring a different approach. Therefore, before undertaking this method, the location, duration, and length of the occlusion and the status of the collateral venous drainage must be considered.
The location of the occlusion dictates the initial approach to access. In most instances, puncture of a patent segment of vein peripheral to the occluded segment is desirable. This provides a good target for the initial puncture and a secure footing for catheter exchanges and venograms (Fig. 10–3). Occasionally, it will be necessary to puncture the occluded vein directly. We reserve this approach for relatively recent occlusions when the vein, distended by thrombus, is easily identified with US.
The duration of the occlusion is important when deciding whether to attempt recanalization. Chronic occlusions are more difficult to cross, with a higher risk of perforation. This is particularly the case with long occlusions. Recent occlusions, whether long or short, are less difficult to traverse; however, it is difficult to predict the success of crossing an occlusion without trying.
The collateral drainage around an obstruction is an important consideration when contemplating a simple recanalization. Peripheral propagation of thrombus is a risk with this strategy and could occlude the collaterals and lead to decompensation of the venous drainage. This is more likely to occur in patients with poor collaterals and an uncontrolled hypercoagulable diathesis. In this situation, a different access site rather than recanalization is appropriate. Alternatively, in some instances, enlarged collateral actually may offer a suitable conduit for catheter placement.10
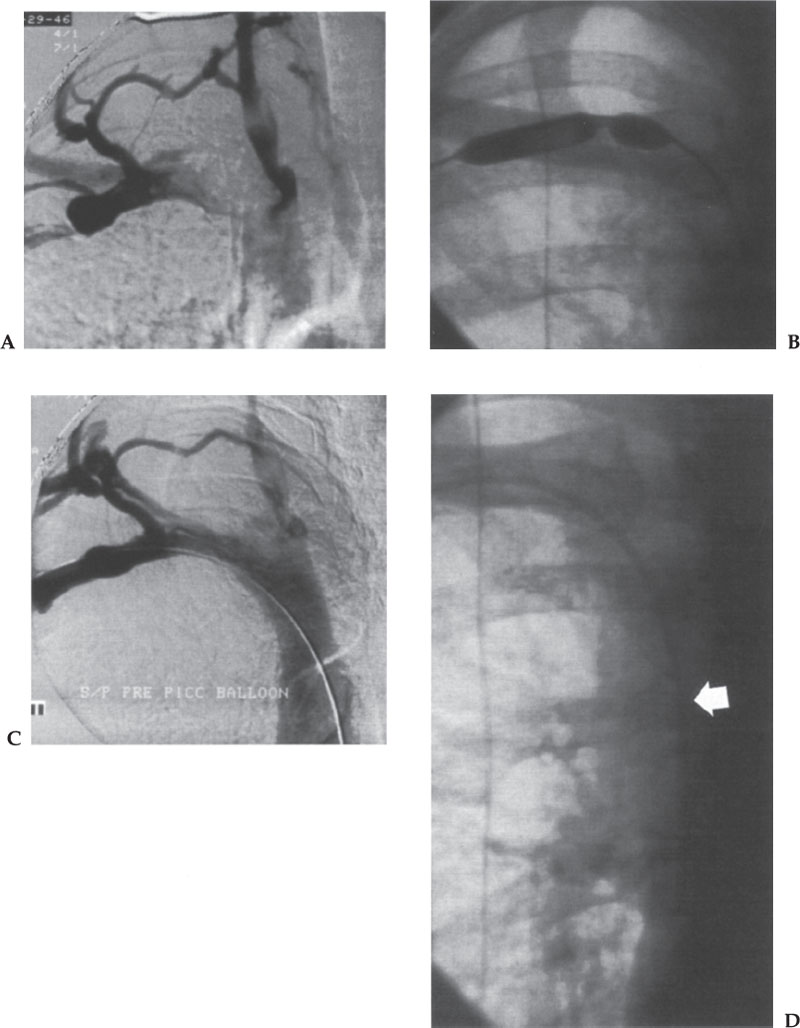
Figure 10–2 Angioplasty of a subclavian vein stenosis during insertion of a peripherally inserted central catheter line. (A) Arm venogram shows stenosis of the right subclavian vein at the junction with the internal jugular vein. Prominent collateral drainage is present. The patient was asymptomatic. (B) Image during the angioplasty shows a tight “waist” in the balloon at the stenosis. (C) Venogram after the angioplasty. The catheter was easily advanced over a hydrophilic guidewire. (D) Successful placement of the catheter (arrow).
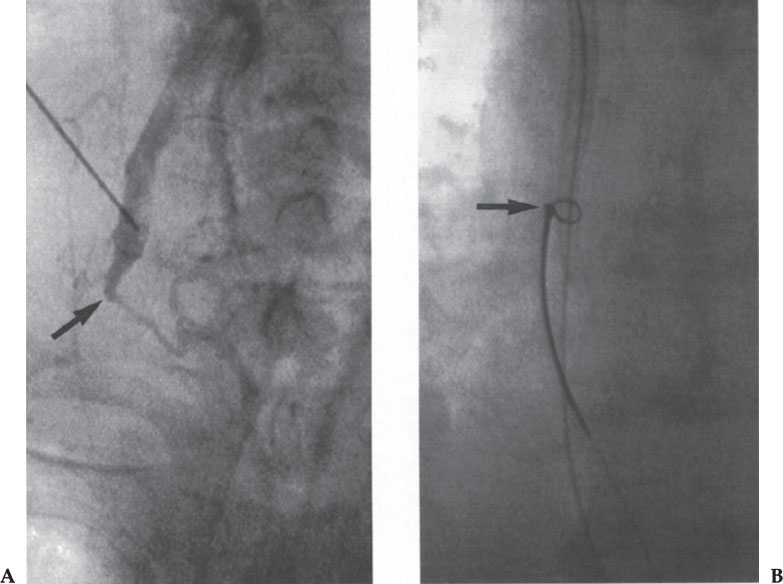
Figure 10–3 Recanalization of an occluded right brachiocephalic vein. (A) Injection of contrast in the right internal jugular vein shows distal occlusion (arrow). This was crossed with a straight hydrophilic guidewire and dilated progressively. (B) After removal of the peel-away sheath, the catheter could not be advanced into the right atrium. A loop snare (arrow) inserted via the common femoral vein was used to pull the catheter tip into the final position.
Ultrasound, venography, and rarely CT can be used to guide the initial access. Micro-puncture kits (Cook, Inc., Bloomington, IN, U.S.A.) that allow puncture with a 21-gauge needle, access with an 0.018-inch mandril wire, and insertion of a coaxial dilator that converts the access to an 0.035-inch or larger system are invaluable for this approach. After initial access is obtained peripheral to the occlusion, an angled hydrophilic catheter such as an H-l or C-2 should be advanced to the site of obstruction. We prefer a 5 French (F) braided catheter (Slip Cath, Cook, Inc.) with a tapered tip. Injection of contrast at this location may reveal a tiny residual lumen (the best possible case) or perhaps a small “nipple” that marks the former lumen. The occlusion is probed with a 0.038- to 0.035-inch hydrophilic guidewire while using the angled catheter to direct the effort. A straight hydrophilic guidewire is preferable because an angled guidewire may enter small tributaries as it is advanced. In difficult cases, progressively stiffer guidewires (including the back end of an Amplatz guidewire) can be used as necessary to initiate crossing the lesion. Sharp recanalization with a needle from a transjugular intrahepatic portocaval shunt (TIPS) kit has been described.11
When antegrade attempts to cross obstruction fail, a retrograde approach, from a femoral vein, should be considered. An H-l or other slightly angled catheter is used in conjunction with a hydrophilic guidewire to cross the lesion. Larger, stiffer catheters such as 6 and 7 F can be useful in the beginning, although frequently 5 F hydrophilic catheters are needed to traverse the lesion completely.
HELPFUL HINT
Long sheaths (40–60 cm) provide support for the catheter while working through the lesion and exchange-length guidewires are essential to avoid losing access during catheter exchanges.
Once through the lesion, the guidewire may be snared through the upper extremity access site and brought out of the arm through a sheath. Ultimately, an exchange-length 0.035-inch Amplatz guidewire should be inserted across the two access sites, bridging the occlusion. This is a very secure situation because the guidewire can be controlled from both ends during the introduction of dilators, sheaths, and catheters (so-called body floss).
When the goal is limited to placing a catheter, the occlusion may be dilated with progressively larger vascular dilators. For large catheters, angioplasty of the entire occluded segment with a 6- to 8-mm balloon results in a channel that will easily accommodate most catheters. More lasting recanalization is rarely necessary in this setting but can be accomplished with placement of a stent immediately before catheter insertion. Prophylactic low-dose Coumadin is strongly recommended in this setting to promote stent patency. Catheter-directed thrombolysis of an acute central venous occlusion, before placement of a long-term central venous access catheter, significantly lengthens the procedure.10
Once the occlusion has been crossed and dilated, the introducer peel-away sheath for the catheter should be placed so that it is completely across the occlusion. This ensures the ability to place the catheter after the guidewire is removed. In some cases, the peel-away sheath provided with the access catheter is not long enough. Either a longer peel-away sheath should be inserted or the catheter placed over one or two guidewires. In the latter case, angioplasty of the occlusion with a balloon several millimeters larger in diameter than the catheter is helpful. Pinching the external end of the peel-away sheath tightly while loading the catheter on to the guidewires reduces the serious risk of air embolism and reduces blood loss. Valved sheaths can be used when inserting ports that are supplied with detached catheters. Occasionally, a snare introduced from below may be needed to pull a catheter into position.
Translumbar Cannulation of the Inferior Vena Cava
Translumbar placement of central venous catheters was first described in 1985.12–17 Infection and occlusive IVC thrombosis rates are less than 5%, respectively, although catheter malfunction is common.14,15 All types of long-term central venous access catheters can be inserted using this approach, including large-bore dialysis and plasmapheresis catheters. Procedural complications such as arterial puncture and retroperitoneal hematoma have been reported.15 Catheter tip migration can occur due to respiratory motion, patient movement, or accidental dislodgment.16 Nevertheless, this access is straightforward, safe, durable, and reliable (Table 10–2).
Patient evaluation begins with an assessment of the skin of the back and abdomen of the right side. Open wounds, surgical drains, infection, or tumor involvement of these areas are contraindications to this approach. Because the catheter is tunneled from the back to the abdomen overlying the anterior aspect of the lower ribs, a clear pathway must exist. Also, patients must be able to cooperate and lie in a decubitus or semiprone position for the procedure. Review of cross-sectional imaging may reveal important information, such as a left-sided IVC.17 An abdominal CT scan with oral and intravenous (IV) contrast or an MRI should be obtained before the procedure is performed if there is any question of IVC patency, unusual caval anatomy, or retroperitoneal pathology.
Table 10–2 Translumbar Inferior Vena Cava Catheter Placement
1. Review prior abdominal imaging studies |
2. Examine skin of right lower back and abdomen |
3. Check coagulation studies, platelets |
4. Consider pigtail catheter in IVC via femoral approach |
5. Position patient left lateral decubitus or partially supine with right side elevated |
6. Wide skin prep |
7. Use long 21-gauge micropuncture needle or translumbar aortography set |
8. Puncture just above right iliac crest 8–10 cm from midline |
9. Advance needle to just anterior to L2–3 to L3–4 interspace |
10. Aspirate blood |
11. Inject contrast to confirm position |
12. Use 0.035-inch Amplatz super-stiff guidewire for dilatation |
13. Tunnel around curve of flank to lower chest/upper abdomen |
14. Locate port pockets over lower ribs anteriorly |
15. Place catheter tip above renal veins, preferably in right atrium |
IVC, inferior vena cava.



