Fig. 2.1
SA-β-gal activity as cellular senescence. (a) Representative photographs of SA-β-gal staining in passage 8 and 15 of human umbilical venous endothelial cells (HUVECs). (b) SA-β-gal-positive staining was observed in atherosclerotic lesions of the intimal side of human coronary artery, which was obtained by autopsy. No staining was detected in the outer surface and normal surface. (c) SA-β-gal-positive staining was observed in atherosclerotic lesions of the intimal side of human thoracic aorta, which was obtained by autopsy. No staining was detected in the non-atherosclerotic area and advanced atherosclerotic area, including the necrotic core and ulcer complicated lesion (Cited from Hayashi et al. 2006)
In NO donors in which tolerance is unlikely to occur, DETA/NO lowered SA-β-gal activation in concentration and time dependency in HUVECs and increased telomerase activation (Hayashi et al. 2006). When eNOS was introduced into HEK293 cells that have no intrinsic NOS, both NO production and telomerase activation increased. Similarly, when introduced into HUVEC, NO production increased, SA-β-gal-positive cells decreased, and telomerase was activated.
2.3.2 Dyslipidemia and Cellular Aging
Increased incidence and severity of risk factors including dyslipidemia and hypertension associated with aging and aging itself have contributed to the saying, “a man is as old as his arteries (word of Sir William Osler, 1891, from Lim and Townsend 2009).” Human LDL values increase in both males and females from approximately 1 year after birth to eventually reach a value four times that of other mammals (approximately 25 mg/dl) (Brown et al. 1980). Incidences of cases with TC of 220 mg/dl or higher have increased by approximately twofold in elderly people from 1980 to 1990. In 1990, approximately 25 % of males and 45 % of females showed plasma TC higher than 220 mg/dl (JAPAN Guideline for Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases 2007). For plasma LDL cholesterol levels, almost the same situation occurred. IHD incidence also increases with age and human serum lipid itself is involved in intimal hypertrophy and atherosclerosis formation.
2.3.3 Diabetes Mellitus and Cellular Aging
HUVEC eNOS protein and NO production decreased with 72 h of hyperglycemia administration (Hayashi et al. 2006). These levels recovered by 30 % with the addition of NOS substrates (l-arginine, etc.) or antioxidants (vitamins C and E) (Hayashi et al. 2005). At a normal glucose concentration, NOS substrates and inhibitors do not affect cellular aging. To state this differently, culture stress alone does not cause eNOS substrate deficiency or uncoupling. Effects were additive, with SA-β-gal-positive cells increasing with high glucose treatment and NOS substrate and antioxidants reducing positive cell count (Hayashi et al. 2008a).
2.3.4 Recent Knowledge
Glucose and insulin are associated with aging and endothelial dysfunction in geriatric diabetic patients: HUVECs and HAECs were cultured for 3–28 days at each glucose concentration, and the effect of insulin on cell function was observed (Miyazaki-Akita et al. 2007). Elevated glucose promoted SA-β-gal activation and reduced telomerase activation. NO metabolites decreased and ROS increased. Although all concentrations of insulin promoted aging at normal glucose levels, under hyperglycemia, physiological concentrations of insulin inhibited telomerase-dependent aging. Both cellular aging caused by eNOS siRNA with normal glucose and effects of physiological concentrations of insulin were NO dependent. Hyperglycemia promoted endothelial cell aging and insulin effects changed depending on the conditions, with NO and reactive oxygen also involved. The potential mechanisms underlying the ability of NO to prevent endothelial cell senescence and the possible changes in the NO-mediated anti-senescence effect under pathological conditions are schematically depicted in Fig. 2.2a, b. Forkhead box O (FOXO) transcription factors are involved in multiple signaling pathways and play critical roles in a number of physiological and pathological processes, including differentiation, proliferation, and survival. FOXO is a mammalian homolog of Daf-16, and activation of Akt leads to phosphorylation of FOXO and thereby inhibits the transcription of antioxidant genes such as manganese superoxide dismutase. Finally, FOXO can affect TERT (telomerase reverse transcriptase) activity by regulating levels of ROS under enough amount of BH4 (tetrahydrobiopterin, Fig. 2.2a). Alternatively, when the signaling pathway such as insulin/IGF-1/PI3-K/Akt is activated, decreased FOXO activity due to phosphorylation leads to decreased FOXO-dependent expression of antioxidant genes (Fig. 2.2b), which is considered to be associated with the potential mechanisms of FOXO-related senescence. It may also contribute to atherosclerosis formation that continues for more than a year with replicative senescence. Intermittent hyperglycemic stimulation also caused cellular aging in a postprandial hyperglycemia model (Hayashi et al. 2010).
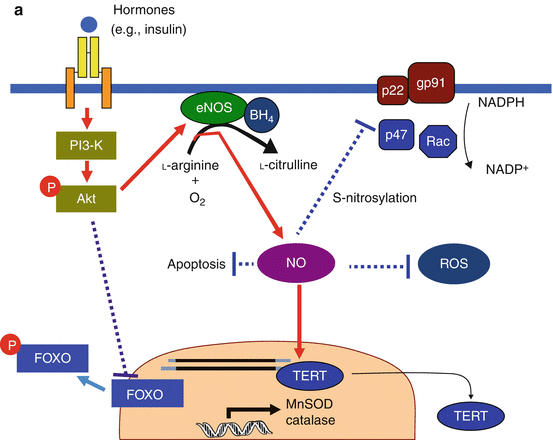
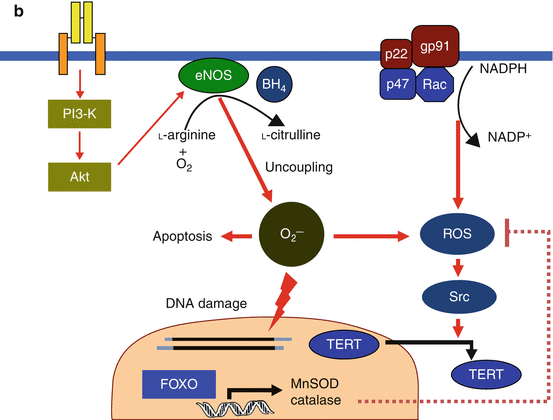


Fig. 2.2
(a) Anti-senescence effect of NO in endothelial cells. Under the normal homeostasis condition, endothelial NO is synthesized from l-arginine and oxygen in a reaction catalyzed by eNOS. Hormones such as insulin or estrogen activate eNOS expression through the PI3-K/Akt pathway and/or direct effect via promoter or stabilization of eNOS mRNA. They may also inactivate NADPH oxidase. NO shows antiapoptotic effects and suppresses ROS production by scavenging directly or preventing NADPH, which would result in depletion of TERT export to the cytoplasm from the nucleus. Activation of the PI3-K/Akt pathway can also downregulate FOXO, leading to decreased FOXO-dependent expression of antioxidant genes. However, with the normal condition of endothelium, the balance between the antioxidant effects of NO and ROS production may be kept well. Solid lines represent positive regulatory pathways. Dotted lines represent negative regulatory pathways. (b) Progress of endothelial senescence under pathological conditions. Both the decrease in eNOS expression by the decline in the hormonal signal with age or insulin resistance and the increase in ROS production by endogenous or exogenous sources cause progression of endothelial senescence by unbalance of NO and ROS. Additionally reduction in BH4 under pathological conditions such as diabetes causes eNOS uncoupling. Under such an uncoupling state, eNOS no longer produces NO and instead generates superoxide. Then, stimulation of the PI3-K/Akt pathway causes not only downregulation of FOXO but also increased eNOS uncoupling, which may result in accelerating endothelial senescence. Solid lines represent positive regulatory pathways. Dotted lines represent negative regulatory pathways (Cited from Hayashi et al. 2008a)
Endothelial cell signal transmission including eNOS is performed in caveolae that cover more than a certain percentage of the cell surface area. This percentage changes in tandem with glucose. When one observes THP-1 cells, macrophage system cells with atomic microscopy, localization of NADPH oxidase changes according to glucose concentration (Hayashi et al. 2007a). When prognosis of late-stage aging was looked at with a range of physiologically active substances and nutrition indices, albumin and (not cytokines of BNP) NO metabolite concentrations were significant biomarkers (Hayashi et al. 2007b). Even with the addition of comprehensive functional assessment of the elderly (physical and mental evaluation including cognitive function and ADL), NO metabolites were useful markers comparable to basic ADLs. In our scientific research for the MHLW, we conducted an observational study of 4,014 diabetic patients with a mean age of 67.4 years and reported that HDL was a risk factor for CVA in late-stage elderly (Hayashi et al. 2008b, 2009). Even now, there is no other treatment for maintaining and raising HDL than exercise, and this is related to the fact that ADL and NO have been recognized in elderly prognosis. HDL and eNOS act reciprocally (Mineo and Shaul 2003).
2.4 Elderly Cardiovascular Disease: Dyslipidemia
Rankings for causes of death change with aging, and in the late-stage elderly, atherosclerosis holds a higher position than malignancies (Besdine and Wetle 2010). It has been suggested that the increased incidence and severity of risk factors including dyslipidemia and hypertension associated with aging as well as aging itself have contributed to the saying, “a man is as old as his arteries” (word of Sir William Osler, 1891, from Lim and Townsend 2009).
2.4.1 Dyslipidemia
High blood total cholesterol (TC) and low blood HDL cholesterol are also independent risk factors for IHD, CVA (cortical branch), and arteriosclerosis obliterans (ASO) in the elderly. The significance of high blood triglycerides as a risk factor for these atherosclerotic diseases in the elderly is unknown. The secondary preventive effects of anti-dyslipidemic pharmaceuticals on IHD have been proven, even in elderly patients, with large-scale trials. Two large-scale trials conducted in Japan reported primary preventive effects of therapy for dyslipidemia on the risk of cardiovascular disease in subjects that included female patients, but the trials only included some early-stage elderly subjects (Mizuno et al. 2008; Yokoyama et al. 2007). In the West, an investigation including late-elderly subjects (Proper et al.) reported its usefulness, but effects were not as pronounced as expected (Heart Protection Study Collaborative Group 2002; Sever et al. 2003; Shepherd et al. 2002).
Looking at age-related changes in lipid metabolism, the elderly have a lower actual lipid intake than younger people and the rate of absorption from the digestive tract increases slightly with age (Saltzman and Russell 1998). The hepatic ability and rate of apoprotein synthesis do not change with age – production rate of hepatic VLDL increases and LDL receptors decrease. LDL activation decreases with age and lipoproteins rich in small dense cholesterol (chylomicron and VLDL remnants) are stationary for a long time in the circulation. LDL catabolism decreases with age and lipid concentration may rise (Matthan et al. 2005). The above changes in serum lipid levels caused by aging increase the atherosclerotic propensity through increased absorption and production.
Serum TC levels have been previously shown to exhibit minimum values in both Japan and America before 20 years of age and increase along with aging. They peak in the 1960s and subsequently diminish gradually. Although recent studies have shown that TC levels decreased in men and women of almost all ages in the USA, the same trend was seen in each age group (Abbott et al. 1998). Annual examinations involving in-class screening of 20,000 people aged from 20 years to approximately 80 years were conducted for 10 years from 1970 (Sekimoto et al. 1983). It was found that both TC and LDL increased in males of all age groups from their late 20s to their 70s. TC and LDL also increased in females from the late 30s and increased dramatically when women were approximately 50 years of age (influenced by menopause). In women in their 70s, results leveled off. Successive changes over 10 years dictate that if the person is 50 years old and born in the 1920s, both males and females will have approximately 200 mg/dl. If the person is 50 years old and was born in the 1960s, this increases by 20 mg/dl. It has long been reported that TC levels in young Japanese subjects are lower than TC levels in US subjects of the same age range and the same trend can also be seen in other age groups. However, now the situation has changed. Japan Ministry of Health Labour and Welfare (MHLW) statistics have also shown the number of cases with serum TC levels of 220 mg/dl or higher to have increased approximately twofold in the elderly over the 10-year period from 1980 to 1990. In 1990, approximately 25 % of males and 45 % of females showed their plasma TC higher than 220 mg/dl (JAPAN Guideline for Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases 2007). For plasma LDL cholesterol levels, almost the same situation occurred. The Westernization of the Japanese diet (high-fat diet, increased number of processed foods, increased calorie intake) has also affected this. There was no great difference in statistical levels reported by the MHLW in 2000 when compared with results released in 1990 (Kuzuya et al. 2002). The MHLW survey also includes examinees of medical institutions, and outcomes may differ from those of screened patients. Even in Japan, the public has become more enlightened about dietary therapy, and cholesterol content has come to be displayed on food packages, like in Western nations. Statins have also come to be more widely used.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


