Fig. 6.1
IMRT plan showing dose distribution in a locally advanced unresectable apical NSCLC
Although no prospective randomized study comparing 3D conformal radiation therapy (3DCRT) to IMRT in lung cancer has yet been published, retrospective studies suggest that IMRT be at least as effective as 3DCRT, in terms of overall survival (median survival time of 1.40 years in the IMRT group versus 0.85 years in the 3DCRT group), distant metastases-free survival and toxicity (hazard ratio of 0.33) [24].
The basic principles of IMRT derives from the fact that a set of intensity-modulated beams from multiple directions can be designed to produce dose homogeneity within the tumor similar to that from conventional radiotherapy but with superior conformity, especially for concave or other complex-shaped target volumes, thereby sparing nearby normal tissues. IMRT attempts to achieve more optimal absorbed-dose distributions by varying the beam intensity (fluency) within each incident beam, usually by subdividing the beam into a number of smaller segments and modulating each to achieve its selected fluency contribution. Furthermore, IMRT treatment planning allows the physician to implement his desired absorbed-dose distribution and the optimization involves a series of parameters to achieve this dose distribution [26].
Management of Physical Uncertainties in IMRT
However, there are potential drawbacks to steep dose gradients, including physical uncertainties regarding ability to deliver the dose to moving targets and to account for potentially changing dose heterogeneity patterns along the beam path. The first of these issues is due to breathing motions. Dynamic IMRT (as opposed to step and shoot IMRT) uses moving leaves while the dose is delivered. As the tumor and the multi-leaf collimator move simultaneously, it is uncertain whether the tumor will actually receive the planned dose, or just remain hidden by the leaves during the whole treatment sequence. After a large number of fractions, this so-called interplay effect, can cause a blurred dose distribution with an increased peripheral beam penumbra, leading to a less conformal dose distribution [26]. Blurring is increased by setup errors and intra or inter-fraction motion. When applied in actual treatment plans, these theoretical findings seem to have a limited impact on dose distribution [26]. This blurring effect may have significant impact incases with large organ motion, where real-time image guidance using tumor gating or tracking are indeed necessary.
This concern may be exacerbated when using flattening filter free (FFF) beams, which are getting more common in the last generation of linear accelerators, especially when dealing with stereotactic irradiation. The flattening filter’s role is to make the photon beam dose distribution uniform at reference depth within the allowed variations and its use significantly reduces the photon dose rate. While FFF beams allow much higher dose rates, which make for shorter treatment with fewer organ motions within the fraction [27], using FFF beams for large treatment fields might raise the question of dose uniformity within the target volumes. When used in dynamic IMRT, FFF beams seem even more susceptible to the interplay effect. This may be partially compensated using increased number of fields and fractions [28].
The impact of tumor and organ motion on dose distribution might be partially compensated for by using respiratory gating or tumor tracking. Additional changes include anatomical changes (patient and target geometry, tumor shrinkage, lung density changes…) over the course of treatment. In dosimetric studies, anatomical changes seem to have a larger effect on actual dose delivery than inter-fractional baseline shifts with an absolute difference in CTVT mean dose over 1 % [29]. This difference might be explained by the fact that respiratory motion is partly taken into account in the PTV margins. These changes may be best assessed by regular 4D-CT scans, measuring both anatomical changes and baseline shifts. As this is technically difficult, as well as time consuming, a mid-treatment 4D-CT scan may be recommended to assess the need for dosimetric adjustment.
Another concern is dose heterogeneity, which is inherent in IMRT. By increasing the number of beams, even though the dose conformity to the PTV may be higher, the dose distribution tends to be more heterogeneous. Adding a homogeneity dose constraint (e.g. standard deviation of the dose distribution less than 3 %, much like what would be seen in 3DCRT) may be used to increase the dose in the PTV while maintaining OAR dose constraints [30].
Low Doses in IMRT
Regarding normal lung irradiation, due to the increase in beam number in IMRT, lung V20 is significantly lower using IMRT in Liao’s retrospective study (37 % using 3DCRT versus 34 % using IMRT) [24]. Other studies have shown similar results of normal tissue sparing using IMRT, for lung and other critical structures. However, because of the increase in beam number, lung V5 is increased in IMRT (57 % versus 65 %) [24–31]. Low doses to a large amount of lung tissue can also result in late toxicity such as lung fibrosis. However, in the retrospective series of patients treated at the MD Anderson Cancer Center, only 5 % of patients demonstrated grade 2 (symptomatic) or higher fibrosis after 18 months [32]. Other toxicities also seem manageable, with under 20 % of grade 3 or higher esophageal toxicity in the MD Anderson patient series, under 10 % of grade 3 or higher dermatitis, and a Karnofsky Performance Status that remained stable in over 60 % of patients [32]. Similar topilot IMRT studies in other tumor types like head and neck tumors, unexpected toxicities may arise along the beam paths, due to the increased number of entry points. High dose voxels can occur in normal tissues, such as vertebra, ribs, or normal lung tissue, as an involuntary consequence of sometimes too harsh dose-constraints. Uyterlinde et al. recently reported a large number of vertebral fractures, in as many as 8 % of patients treated with IMRT and concurrent chemotherapy for NSCLC [33]. Although these results come from a small patient sample, they must be viewed as an incentive to be aware of unexpected toxicities, as with any new technique. Overall, even though most of the data on IMRT in lung cancer remains retrospective or purely theoretical, it seems to be a promising technique. Clinical outcomes with IMRT in locally advanced lung cancer are reported as good as or better than with 3DCRT, and with fewer and less severe toxicities.
Patterns of Care with IMRT
SEER analyses [34] suggested an increasing use of IMRT in stage III lung cancer between 2001 (0.5 %) and 2007 (14.7 %). Such increase is not related to clinical parameters but was rather observed in freestanding centers (as opposed to university hospitals) in an attempt to value (with Medicare reimbursement) high technology radiation therapy. This SEER-based study confirmed that the risk of radiation-induced pneumonitis and esophagitis is not increased with IMRT.
While IMRT is not approved in lung cancer (except for sulcus, paraspinal and some paracardiac tumors), it is performed in up to 100 % in some institutions.
Finally, patterns of practice are rapidly changing and the level of evidence for IMRT is increasing. 4D planning appears necessary with tumor motion monitoring limiting amplitudes to ≤1 cm and reduced CTV/PTV margins. Large tumors, in close proximity of OAR, such as the spinal cord, brachial plexus, esophagus, mediastinum, might be treated more efficiently with IMRT than with just conformal irradiation. Attention to cold and hot spots and attempts to limit the low doses to the normal lung (limited field number?) as well as carefully accounting for tissue heterogeneity/dose calculation algorithms/affected beamlets should be encouraged. Strict QA programs verifying reproducibility in all steps of the planning process to dose delivery are necessary. Of note, several technological advances may be analyzed indistinctly. For example, randomized studies are ongoing to determine the benefit of IMRT using 4DCT planning vs. 3DCRT (ClinicalTrials.gov: NCT00520702) and of image Guided Adaptive Conformal Photon vs. Proton Therapy in the concurrent setting (ClinicalTrials.gov: NCT00495170). Prospective randomized clinical trials are still needed to back up these findings. Several prospective studies are presented on the NCI clinicaltrials.gov website (NCT00921739, NCT01836692, NCT01166204, NCT00497250, NCT01617980, NCT01577212, NCT00938418, NCT00690963, NCT01411098747, NCT01429766, NCT01822496, NCT01912625, NCT01024829, NCT01059188, NCT01391260, NCT01494415, NCT01580579).
Dose
Dose Escalation
The standard dose regimen with standard 2D radiation therapy was established by the RTOG 7301 at 60 Gy in 2 Gy/fx [35]. However, median survival was about 10 months, with 3-year survival <10 %. Several other studies conducted in the 1980 sattempted to increase the dose to improve local control. They showed a strong correlation between total dose and local control and overall survival of locally advanced NSCLC [36]. The randomized phase I/II RTOG 8311 trial tested dose escalation (60, 64.8, 69.6, 74.5, and 79.2 Gy) using a hyperfractionated 1.2 Gy twice-daily regimen in 848 patients. The 69.6 Gy group had better outcomes than the lower doses groups. It was assumed that doses above 70 Gy did not increase survival further as a result of radiation-induced lung toxicity because of the volume of normal lung tissue irradiated [37]. Later studies increased the total dose with conformal irradiation [2] from 65 to 102 Gy with acceptable acute grade 3–4 lung and esophageal toxicity rates (4−12 %) with little reporting of late toxicities [38, 39]. Compared to historical results, dose escalation protocols produced higher local control rates (50−60 %) than standard radiotherapy, with 2-year survival rates around 40 %. The higher esophageal toxicity did not result in significant radiation protraction or interruption. Acute heart toxicities occurred in 8 % of a cohort of 50 patients treated with total doses up to 74 Gy [38, 39]. A retrospective study conducted by the Memorial Sloan Kettering in 2007 included 82 patients with inoperable NSCLC Stage I-IIIB [40]. A dose ≥80 Gy was used conformal radiation therapy with sequential chemotherapy. For stage III, 5-year local control and survival rates were 39 and 31 %, with advantage benefit of dose escalation consistent with other studies. Kong et al. [41] showed a benefit of dose escalation on 5-year survival with corresponding improvements with dose in local control. Further, phase I studies like one published by Rosenzweig et al. including 104 patients (65 % stage III and 6 % recurrent) receiving conformal irradiation without elective nodal irradiation showed significantly improved overall survival with dose ≥80.0 Gy, with induction chemotherapy given to 16 % of patients and no concurrent chemotherapy [42]. Crude late pulmonary toxicity was 7 %. The maximal tolerated dose with a normal tissue control probability (NTCP) constraint of 25 % was 84.0 Gy. The RTOG 84–07 (1984–1989) concomitant boost phase I/II trial used 45 Gy on large fields including primary + regional nodes and a 18 Gy boost on primary and involved nodes only to a total dose of 63 Gy. Dose was escalated from 63 to 70.2 Gy/5.5 or 5 weeks [43]. Acute and late toxicities increased within reasonable rates but survival was not statistically different between arms. For patients with Stage III/KPS > 70/no weight loss (i.e. eligible for CALGB 8433) 2-year overall survival was about 20 %.
Later trials, like the CALGB 30105 phase II trial, used dose-intense chemoradiation combinations. The CALGB used induction chemotherapy followed by concurrent chemoradiation in stage III NSCLC with dose-escalated thoracic conformal radiotherapy (74 Gy, once daily, 2 Gy per fraction) in both arms [44]. Patients were randomized between carboplatin/gemcitabine, which arm was closed prematurely due to an unacceptably high rate of grade 4–5 pulmonary toxicity attributed to radiosensitization by gemcitabine. The carboplatin/paclitaxel arm yielded a median survival of 24 months with a 12 % rate of grade 3 or higher pulmonary toxicity [44]. These promising results formed the basis for the experimental arm of the phase III RTOG 0617 trial.
The Randomized Phase III RTOG 0617 compared Standard- Dose (60 Gy) Versus High dose (74 Gy) Conformal Radiotherapy with in patients with Stage IIIA/IIIB Non-Small Cell Lung Cancer. The 2 × 2 factorial design consisted of weekly concurrent carboplatin–paclitaxel chemotherapy with randomization between 60 Gy or 74 Gy, and two cycles of Concurrent and Consolidation Carboplatin/Paclitaxel with or without cetuximab. The high dose arm was closed prematurely after showing 85 documented events and a likely detrimental effect with high dose radiotherapy, which correlated with patient-reported deteriorated quality of life [104]. Updated analysis after 207 events demonstrated a significant increased risk of death in the high dose arms (median survival 28.7 months (60 Gy arm) vs. 19.5 months (74 Gy), p = 0.0007), with a 37 % increased risk of local failure in the high dose arms. There were also a trend for more treatment-related deaths in the high dose arms (10 vs. 2) [105]. Why the RTOG 0617 trial is not consistent with the results of previous phase II trials remains investigational and methodological issues are being looked for. While the exact reasons for such deleterious effects are yet unknown, it was shown that these results are consistent with patient-reported quality of life (ASTRO 2013). Hypotheses include accelerated repopulation due to protracted irradiation, increased reported protocol deviations, under-reporting of lung and cardiac treatment-related deaths in patients who received excessive radiation dose to heart and lung in the high dose arms, and a possible negative interaction between cetuximab and high dose radiotherapy.
Altered Fractionation
Altered fractionation regimens using concomitant boost or hyperfractionated and/or accelerated radiation therapy have been conducted in locally advanced lung cancer. The CHART (Hard hyperfractionation through Continuous Hyperfractionated Accelerated RadioTherapy) trial has demonstrated that overcoming the accelerated repopulation effect by delivering 54 Gy three times daily in 12 consecutive days results in a significant survival benefit compared to conventional regimens [45]. However, logistics of delivering irradiation thrice daily for 12 days limited its use in routine practice. Alternatively, many combinations using either different timings or different drugs were subsequently used to improve local control, distant control and survival. Several phase II trials like the RTOG 9204 (twice daily irradiation with chemotherapy) showed improved local control at the expense of higher esophageal toxicities [46]. More recently, an individual patient data meta-analysis of 10 randomized trials (2,000 patients) comparing hyperfractionated and/or accelerated radiotherapy to conventional fractionation has confirmed the advantage of altered fractionation, increasing 5-year survival rates by 3 %. Dose escalation and dose redistribution based on functional imaging is at the heart of the EU Framework Program 7 (FP7) funded PET Boost trial (ClinicalTrials.gov Identifier: NCT0102482). The overall treatment dose is escalated by increasing the dose-per-fraction until specified dose constraints are met. The patients are randomized to receive the standard regimen (66 Gy given in 24 fractions of 2.75 Gy) with either an integrated boost to the primary tumor as a whole or to the 50 % SUVmax area of the primary tumor based on the pre-treatment FDG-PET CT scan.
Dose Volume
If local control may be improved by radiotherapy dose escalation according to several studies, toxicity, including esophageal toxicity, and more particularly pulmonary toxicity seems to be related to radiation volume. In locally advanced non-small cell lung cancer, eliminating elective nodal irradiation allows to maximize tumor dose and minimize normal tissue toxicity in combined modality treatments. The use of staging modalities such as positron emission tomography allows encompassing the tumor volume with more accuracy. Several retrospective, randomized phase III studies and a meta-analysis of 1,705 patients from 4 RTOG trials (7811, 7917, 8311, 8407) have confirmed that involved-field irradiation results in a regional nodal failure rate of less than 10 % [47–49] and suggest that it can be performed in routine practice. To that extent, PET CT is highly recommended for suitable diagnostic work up and pre therapeutic staging [50].
Multimodal Imaging for Radiation Therapy Planning and Dose Painting
PET CT in Radiotherapy Planning
PET CT has emerged as an important imaging modality for radiotherapy planning in locally advanced lung cancer as shown in Fig. 6.2 [50, 51]. It helps distinguish between tumor and atelectasia and allows more accurate staging than CT [52, 53]. This results in about a third of patients not receiving the planned curative irradiation and may result in better outcomes in curative disease on PET CT even without dose escalation [54]. However, such data must be interpreted with caution as they are probably a consequence of stage migration. Another study assessed staging changes on hybrid PET/CT scans repeated within 120 days of an initial staging PET/CT [55]. Radiation treatment planning based on repeat PET CT identified significant upstaging in more than half of patients. For a subset of patients who underwent both scans on the same instrument, SUV velocity predicted upstaging, but the difference between those upstaged and those not was statistically significant.
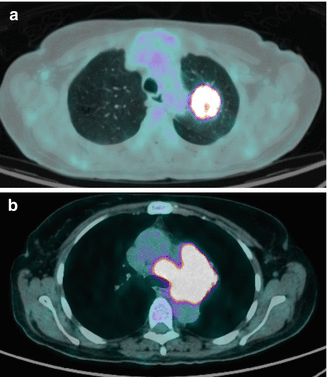

Fig. 6.2
PET CT for radiation therapy planning
Accounting for Hypoxia in RT Planning
Hypoxia has consistently been associated with tumor radioresistance and tumor angiogenesis. Blood flow and volume are both indirect measures of tumor angiogenesis and can be assessed by perfusion studies. Several studies however suggest that there is no correlation between perfusion parameters, using Dynamic contrast-enhanced CT (DCE-CT) that quantifies vasculature properties, and metabolic activity using PET CT in large tumors [54]. Both imaging modalities are however able to show intra-tumor heterogeneity.
An extensively investigated tracer for visualization of tumor hypoxia by dynamic PET-CT is fluorine-18-labeled fluoromisonidazole (18F-FMISO; half life 110 min). Preclinical and clinical studies have revealed a correlation between oxygen measurements and 18F-FMISO uptake. Small preliminary studies demonstrated an association between high tumor-to-muscle FMISO uptake ratios and risk of relapse as well as prediction of outcomes based on FMISO uptake decrease on repeat FMISO PET CT. The HIL trial (Heidelberg team, NCT01617980) currently investigates the correlation between 18F-FMISO PET-CT and functional MRI for tumor hypoxia imaging in patients with inoperable stage IIINSCLC, treated with 4D-CT based IMRT. Other tracers, such as the [(18)F]HX4 is a promising hypoxia PET-tracer, are being studied [56]. In a series of 15 patients, the majority of NSCLC lesions showed considerable [(18)F]HX4 uptake. The heterogeneous uptake pattern was stable between 2 and 4 h with PET imaging at 4 h being superior.
Stereotactic Irradiation as a Boost on Hypoxic Areas
Most experience with stereotactic irradiation is based on limited stage disease management, for inoperable patients. Its use has recently expanded for the management of operable early stages in comparison with surgery (several ongoing randomized phase III trials). The definitions of stereotactic irradiation are various as are the equipments to deliver the dose stereotactically (often in the hypofractionated mode) and the methods used for motion management. There has been limited room for stereotactic ablative radiation therapy (SABR) in the management locally advanced NSCLCC for such clear reasons as tumor bulk (potentially deteriorated coverage, red shell constituting a danger zone on normal tissues outside the PTV). However, SABR is increasingly used either as consolidative planned boost (+/− dose escalation) [57] or as a stereotactic boost for residual disease (or in the recurrent setting). Preliminary retrospective data in patients with stage III disease including N2 disease suggest that a risk adaptive systematic dose escalation strategy with SABR (25 Gy in 5 fractions following 50 Gy conformal irradiation) following external beam radiotherapy is feasible [57]. Such systematic boost strategies may be particularly interesting to boost hypoxic regions. However, moderate to severe acute toxicities were encountered in about 25 % of patients, emphasizing the need for optimal patient selection for hypofractionated SABR. Another study prospectively investigated the role of SABR to deliver a boost on residual disease [58]. Patients without metastatic disease and with radiologic evidence of limited residual disease (≤5 cm) within the site of the primary tumor and good or complete nodal response after standard chemoradiation to a target dose of 60 Gy were considered eligible for an SABR boost. The total combined dose biological equivalent dose was >100 Gy to the residual primary tumor, in two 10 Gy-fractions for peripheral tumors, or three 6.5 Gy- fractions for central tumors according to the RTOG protocol 0813 definitions. After a median follow-up of 13 months, out of 33 patients, four patients developed acute grade 3 radiation-induced pneumonitis, and 1 developed late and persistent grade 3 pneumonitis. At the time of analysis, the actuarial local control rate at the primary tumor site was 83 %. Linear accelerator-based SBRT for dose escalation of limited residual NSCLC after definitive CRT was feasible and did not increase the risk for toxicity above that for standard radiation therapy. Proper daily intra-fractional IGRT methods are necessary and tumor tracking should be recommended whenever possible to best account for intra-fraction movements [59], especially when hypofractionation is used.
Several phase I/II clinical trials currently investigate the role of stereotactic irradiation in locally advanced non-small cell lung cancer (NCI clinicaltrials.gov : NCT01657617, NCT01656460, NCT01746810, NCT01300299, NCT01463423, NCT01051037, NCT01899989, NCT01781741, NCT01345851, NCT00945451, NCT01711697, NCT01543672).
Future Drugs
Pemetrexed
Pemetrexed inhibits enzymes involved in nucleotide synthesis by acting as afolate competitor. Compared to gemcitabine, pemetrexed, in association with cisplatin, increased overall survival in metastatic non-squamous NSCLC [60]. Preclinical studies have shown that pemetrexed is a potential radiosensitizer with radiosensitization depending on drug concentration and tumor type, with an enhancement ratio as high as 1.6 in the LXI lung tumor cell line [61]. Several phase I and II studies have demonstrated that combining pemetrexed with concurrent radiation only yielded moderate toxicities [62–64], was efficient in locally advanced stage III NSCLC and can be safely given at full systemic doses with thoracic radiation therapy [65, 66]. The negative CALGB 30407 trial included squamous NSCLC where activity of pemetrexed is known to be limited [66]. Subsequent trials only included non-squamous NSCLC. The PROCLAIM phase III trial started in 2008, and compared cisplatin plus pemetrexed to cisplatin plus etoposide with concurrent 66 Gy thoracic radiation therapy followed by consolidation chemotherapy [67]. Its aim is to determine if concurrent pemetrexed/cisplatin would translate into a survival benefit. The study enrolled nearly 600 patients. There was a similarly high dose-intensity in both arms, and preliminary results demonstrated significantly lower incidences of overall adverse events and some toxicities including granulocytopenia and infections in the pemetrexed/cisplatin arm [106]. Full presentation of the study results including potential late toxicities is awaited.
More recently, in 2010, the French Thoracic Oncology Group (IFCT) started the IFCT 0803 phase III trial, comparing cisplatin plus pemetrexed with concurrent 66 Gy thoracic irradiation, with or without cetuximab [68]. This “best of” trial combined all up-to-date advances together, i.e. chemoradiation, induction chemotherapy, high dose irradiation (66 Gy), pemetrexed, cetuximab, and a platinum-based doublet. The IFCT 0803 trial just terminated accrual of 106 patients. Garrido et al. recently presented the data of the pemetrexed-cisplatinum (500 mg/m2, 75 mg/m2 d1 q21d) induction and chemoradiation phase II study in non-squamous NSCLC. Progression free survival (1-year rate of 51 %) was similar to that observed in other cisplatinum-based induction and concurrent protocols. The overall response rate was 59 % with 13 % having progressive disease. Dose-intensity was maintained in 71 % of patients. Grade 3–4 toxicities (esophagitis, neutropenia) during the chemoradiation phase were around 10 %, which is generally considered acceptable for locally advanced NSCLC undergoing radical chemoradiation.
Cetuximab
Cetuximab is a monoclonal antibody targeting epidermal growth-factor receptor (EGFR). There have been suggestions, in the FLEX phase III for example, that patients who had a higher expression of EGFR in their tumors (as measured by immunohistochemistry) had a better response than patients with low expression [69]. There was also a strong rationale from preclinical studies [67] and the RTOG 0324 study, to add cetuximab to chemoradiation. Preliminary results from the RTOG 0617 trial at the 15th World Conference on Lung Cancer (Abstract PL03 2013) however suggest that there is no clinical benefit from the addition of cetuximab to chemoradiation in patients with unresectable stage III NSCLC.
Furthermore, the addition of cetuximab is associated with increased toxicity. The cetuximab analysis was carried out on 465 patients, with a median follow-up of 19 months. Weekly cetuximab was added to weekly chemotherapy with carboplatin and paclitaxel, given concurrently with radiation. Median overall survival for patients treated with chemoradiation with or without cetuximab was 23 months, the 18-month overall survival rate 60 %, and median progression-free survival 10 months in both arms. Overall combined adverse events were reported by 85 % of patients treated with chemoradiation with cetuximab vs 70 % without cetuximab, while overall non hematological adverse events were reported by 71 % vs. 51 % of patients, respectively (p <0.0001).
New Targeted Therapies and Other Therapeutics
Patients with EGFR-mutated locally advanced NSCLC have lower local recurrence rates following radiotherapy and/or chemotherapy, raising the hypothesis that EGFR mutation might confer sensitivity to some EGFR inhibitors in a way that is synergistic with irradiation [70, 71]. Tolerance studies have shown that standard dose of EGFR inhibitors such as erlotinib or gefitinib could be given during chemoradiation without significantly increasing toxicities [72, 73]. However, so far, most studies investigating the use of EGFR-tyrosine kinase inhibitors with chemoradiation have been rather disappointing, with survival rates lower or close to those seen with chemoradiation alone [73–77]. In particular, the SWOG S0023 study, comparing gefitinib and placebo after cisplatin/etoposide/63 Gy and consolidation docetaxel in stage III NSCLC, showed an increased mean survival time in the placebo arm (35 versus 23 months in the experimental arm), leading to premature trial closure [74]. Similarly, the use of gefitinib after resection of stage III NSCLC was not associated with increased survival [78]. These study results suggest that the use of EGFR-TKI in an EFGR mutation unselected stage III NSCLC population is not warranted and future study should focus on the integration of EGFR-TKI to chemotherapy in patients with activating EGFR mutations.
Available evidence clearly indicates that the mutational landscape, including EGFR mutations [79–86] and ALK translocations [87, 88], of non-small cell lung cancers is relevant to therapeutics to yield personalized medicine.
One recent breakthrough was the discovery of chromosomal rearrangements of the anaplastic lymphoma kinase gene (ALK) in non small cell lung cancers, which can be targeted using oral ALK inhibitors such as crizotinib. Crizotinib was shown superior to standard chemotherapy in patients with previously treated and untreated, advanced non-small-cell lung cancer with ALK rearrangement [107]. Further, preliminary retrospective data suggest that crizotinib not only can be delivered during irradiation but also yields interesting control rates in oligoprogressive lung cancer treated with chemoradiation [89, 90]. Clinical trials in stage III ALK positive NSCLC would be of interest, although their conduction is a challenge due to the low incidence of this rare molecular alteration comprising only 4 % of NSCLC patients. A randomized phase II trial in molecularly selected patients with stage III NSCLC is currently ongoing (RTOG1306).
Anti-angiogenics such as bevacizumab have shown promises in metastatic setting when combined with chemotherapy in NSCLC and has become a first-line therapy in many facilities [91, 92]. However, when used in a radiotherapy setting, bevacizumab is associated with major toxicities, including tracheo-esophageal fistulae, pulmonary hemorrhage, particularly in squamous histology tumors [93, 94], with no survival benefit. As such, there are currently no ongoing trial using bevacizumab concurrently with radiotherapy with lung cancer and its use should be avoided in this setting. Others anti-angiogenics have been assessed in association with chemoradiation or radiation therapy alone in NSCLC. The use of the anti-angiogenic agents (AE-941 or thalidomide) was associated with increased toxicity, mainly thrombo-embolic events, but did not increase survival [95, 96].
Many other targeted agents have been tested in clinical trials, such as efaproxiral, which reduces hemoglobin oxygen-binding affinity, increasing tissue pO2, as a mean to overcome tumor hypoxia. In this study, efaproxiral, combined with radiotherapy after induction chemotherapy resulted in 20.6 months survival, with low toxicity incidence, which makes it a promising drug [97]. A new vaccine to MUC-1, L-BP25, has shown great promise in stage IIIB and IV NSCLC in a phase II trial, with an increase in median survival (30.6 versus 13.3 months) in stage IIIB disease [98]. Based on these results, the phase III START trial randomized 1320 patients with unresectable stage III NSCLC who had disease control after first line chemo-radiotherapy to receive either L-BP25 (Stimuvax) or placebo after priming with cyclophosphamide 300 mg/m2 [108]. The primary endpoint overall survival was not met (25.6 vs 22.3 months, HR 0.88, p = 0.12). However, when analyzing the large subgroup of patients (n = 806) who have received standard concurrent chemoradiotherapy as opposed to sequential treatment, overall survival was increased in the vaccination arm by almost 10 months (30.8 vs 20.6 months, HR 0.78, p = 0.016). Following this encouraging observation a phase III study (START 2) including only patients treated with standard concurrent chemoradiotherapy has been initiated.
Another phase III study investigating the use of GV 1001, a telomerase peptide vaccine, after chemoradiation is planned, following a phase II study showing a significant increase in overall survival (19 versus 3.5 months) in immune responders [99].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


