Fig. 61.1
Bulla of the apex in white light (a) and after inhalation of fluorescein (b) (Courtesy of Prof. Marc Noppen)
Another technique applied recently to investigate bullous or emphysematous lesions of the lung parenchyma is infrared thoracoscopy [13]. After general anesthesia and double-lumen intubation, the lung is observed under normal white light and then observed under infrared thoracoscopy with intravenous injection of 3.0 mg/kg indocyanine green. The infrared thoracoscopic images are analyzed with Lumina Vision (Mitani Co, Fukui, Japan) [13].
The lung lesions are demonstrated in white, whereas normal lung tissue was imaged in blue, under infrared thoracoscopy. Also, small bullous lesions are detectable with infrared thoracoscopy because of its clearer visualization compared with white light thoracoscopy. Quantitative color-density analysis revealed a marked decrease of indocyanine green intensity, reflecting decreased blood flow of bullous lesions [13]. After injection of indocyanine green, infrared thoracoscopy showed that the area of normal perfusion changed to blue, whereas the area at which perfusion was absent remained white [14]. The transition zone between colors was distinct, and the blue stain remained visible during the marking procedure. Three-dimensional computed tomographic analysis indicated that the marking separated the target segmental bronchus from the adjacent one. Detailed macroscopic and microscopic study confirmed that the marking corresponded to the intersegmental line [14].
Both techniques are a step forward in the understanding of the pathophysiology of primary spontaneous pneumothorax by identifying those lesions otherwise undetectable with the white light technique [6].
Thoracoscopy in Pleural Infection
The place of thoracoscopy in the treatment of pleural infection is not clear. An unsolved issue in the management of patients with pleural infection is whether those patients should undergo early thoracoscopic or simply classical treatment. Thoracoscopy is an alternative to thoracotomy because it allows the mechanical removal of infected material leading to lung reexpansion (Fig. 61.2). Furthermore, the ability to perform pleural biopsies allows the diagnosis of any underlying disease helping the diagnosis of pleural malignancies or other occult infections causing pleural effusion (Fig. 61.3). Mortality in Western countries is about 15 %, while up to 40 % undergo surgical drainage, either video assisted or thoracotomy, with pleural decortication [15]. The goal of pleural infection therapy is to heal the patient, to reduce mortality and morbidity overall hospital stay, and the need of surgical drainage and pleural debridement. Classical treatment associates according to the stage, antibiotics with or without chest tube drainage. The use of fibrinolytics in the treatment of pleural infection is another point of discussion; fibrinolytics have shown to be beneficial in patients with complicated parapneumonic effusion [16], yet their place in empyema is questionable.
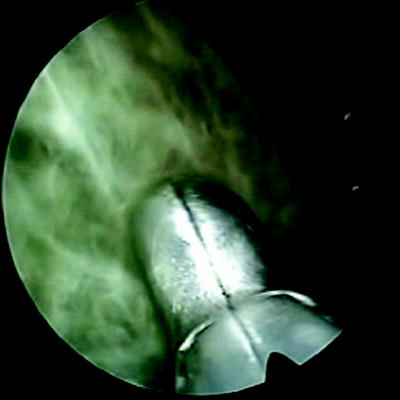
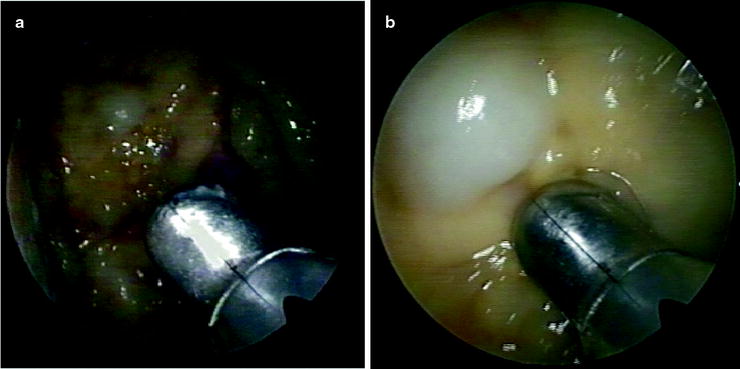

Fig. 61.2
Thoracoscopic view of complicated parapneumonic effusion (multiple loculations)

Fig. 61.3
Staphylococcal empyema in a patient with SCLC. (a) Thoracoscopy revealed multiple metastatic nodules of the parietal pleura (b)
For medical thoracoscopy in pleural infection, it is mandatory to choose the point of entry by ultrasonography, to identify the point where the pus collection is largest and the position of the diaphragm, which is often elevated [17]. Once in the pleural space, loculations must be opened with removal of the fibrinopurulent membranes, aspiration of the liquid, and washing with saline solution. Finally, biopsies must be taken systematically for diagnosis of possible underlying condition favoring pleural empyema (Fig. 61.3). A large-bore chest drain is introduced, better under visual control, to drain pleural cavity and remove dense and viscous pus or fibrin debris (Fig. 61.4) [17].
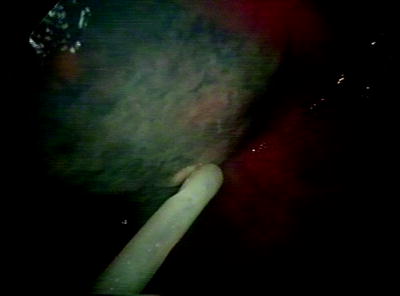

Fig. 61.4
Placement of chest drain under direct vision after thoracoscopic treatment in a patient with empyema
Reports of thoracoscopy in pleural infections are mainly related to empyema and are principally surgical. They generally describe favorable results, with primary success rates (complete recovery without the need for subsequent or conversion thoracotomy) of 60–100 %. The best results were obtained when the method was applied early in the course of the disease [18]. However, few patients were included in those studies to draw firm conclusions. All studies agree on the advantages of thoracoscopy over thoracotomy because of the less invasive technique, in pain, costs, hospital stays, and cosmetic results. A number of advantages are highlighted for medical thoracoscopy [17]. It is mini-invasive, has lower costs compared with VATS, and is useful in the treatment of patients with comorbidities. Complications are strictly related to case complexity and are mainly represented by air leaks, which are sometimes prolonged, and by bleeding, with incidences of between 16 % and 0 % [17]. Deaths were described in patients with comorbidities [17].
Although early minimal intervention with medical thoracoscopy has shown excellent results in recent reports [19], the need for controlled large phase III trials to further define its place in the treatment of pleural infection is requested. Indeed, the few studies reporting results with small number of patients included are not sufficient to draw firm conclusions.
Thoracoscopy in Sympathectomy
Thoracoscopic sympathectomy techniques are currently standard approaches for sympathectomy. Thoracoscopic sympathicolysis is defined as the anatomical interruption of the thoracic sympathetic chain by thoracoscopy. The level of interruption depends upon the indication and the desired therapeutic effects. Essential hyperhidrosis (palmar, axillar, facial) is treated by interrupting T2 and T3 dorsal sympathetic ganglia [20]. Indications for thoracoscopic sympathectomy might include facial flushing, vascular disorders of the upper limbs (Raynaud’s phenomenon, acrocyanosis, arterial insufficiency, Buerger’s disease), causalgia, thoracic outlet syndrome, and cardiac disorders, such as long QT syndrome and chronic pancreatic pain syndromes [17]. Alternatives of thoracoscopic sympathectomy are thoracotomy interruption and percutaneous ablation. However, both techniques are tending to be abandoned, the first because it is invasive and the second because of lower efficacy and higher complication rates [21].
Technique is based on unilateral, or one-time bilateral according to indication, three-entry port thoracoscopy using single-lung, double-lumen ventilation. At the present time, thoracoscopic sympathetic intervention is performed in a 1-day setting, under general anesthesia. The sympathetic chain is located, and electrocautery interruption is performed [20, 21]. Thoracoscopy sympathicolysis can safely be done by trained interventional pulmonologists.
Short – and long-term results are excellent in hyperhidrosis patients. Relief of palmar, axillar, and/or facial sweating is obtained in 90–100 % of cases [20]. Recurrence rates vary between 5 % and 10 %, but repeat interventions are often successful. Complications are rare (<1 %), include Horner’s syndrome, pneumothorax, and hemorrhage, sometimes necessitating conversion to thoracotomy. No procedure-related mortality has been reported [20]. Compensatory sweating occurs in the majority of patients after sympathetic interruption and may be related to the level of interruption (T2 interruption increasing its likelihood) and extent (e.g., extensive-level interruption increasing its likelihood). Patients should be extensively informed about the probable occurrence of compensatory sweating. In general, however, this is considered not more than a nuisance and does not affect overall patient satisfaction. However, a small percentage (1–2 %) of patients regret the intervention afterward [22].
Thoracoscopic sympathectomy is a minimally invasive intervention for patients with a variety of autonomous nervous system disturbances. Well-selected patients can be helped by this procedure, performed by either surgeons or pulmonologists. Short – and long-term results are excellent, complications are rare, and side effects are usually limited to a degree of compensatory hyperhidrosis.
Thoracoscopy in the Diagnosis of Lung Diseases
Surgical thoracoscopy under general anesthesia is the method of choice for the diagnosis of parenchymal lesions especially in patients with diffused lung disease (DLD), when bronchoalveolar lavage (BAL) and transbronchial biopsy (TBB) have failed [23]. Lung biopsy by such procedure provides large specimens that are virtually identical to those obtained by thoracotomy [24]. However, multiple incisions, costly single-use instruments, and general anesthesia with single-lung ventilation are required. Moreover a conversion from thoracoscopy through mini-thoracotomy is not uncommon due to technical difficulties (deep lung biopsies) and/or complications such as prolonged air leak and/or bronchopleural fistula in case of deep lung biopsies. Thus, lung biopsy under surgical video-assisted thoracoscopy, even in ambulatory patients, is not an entirely benign procedure. Furthermore, it seems that the site, size, number, and laterality of the biopsies in patients with DLD have no definite influence on diagnosis. Careful selection of specific thoracoscopy lung biopsy techniques could result in increased cost-effective diagnosis of pleuropulmonary disorders, less parenchymal lung damage, and lesser morbidity (Table 61.1).
Table 61.1
Comparison of thoracoscopy (VATS) to open thoracotomy for lung biopsy
Thoracoscopy | Thoracotomy | |
|---|---|---|
Intervention time | 45 min | −1 h |
Hospitalization | 2–4 days | 3–5 days |
Drainage | 1–3 days | 2–4 days |
Biopsy size | 2–7 cm | >5 cm |
Morbidity | 5–10 % | 7–12 % |
Cost (Euros) | 1,500 | 1,200 |
Despite encouraging published results in the literature reporting high diagnostic yield of thoracoscopy in patients with DLD using electrocautery lung biopsy [25, 26], most pulmonologists do not use this technique in the diagnostic work-up of DLD. Furthermore, experimental studies showed that multiple small (less than 5 mm) electrocautery lung biopsies were of comparable quality compared to single wedge biopsy specimens obtain by endoscopic stapling [27]. However, many pulmonologists probably still fear the use (and the complications) of pleuroscopy in the diagnosis of lung parenchyma lesions, while many pathologists still have to become acquainted with the smaller amount of tissue compared to the larger surgical samples [25]. The yield could be significantly improved by using under CT a needle or a wire as a hook, to localize lesions before thoracoscopy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


