Fig. 11.1
Growth variation in infants with single ventricle. Variation in weight for age z-score changes, by surgical site caring for the patient, measured from stage 1 discharge to presentation for stage 2 palliation (Reprinted from Anderson et al. [34], copyright 2012, with permission from Elsevier)
All infants and children with congenital heart disease who are at risk for growth failure require close monitoring of anthropometric measurements during periods of increased risk for growth failure. Early detection of growth failure is essential to assure that appropriate nutritional interventions are accomplished with sufficient time to improve growth before subsequent surgical procedures are undertaken. It is recommended that weight, length/height, head circumference as well as weight-for-age, length-for-age, head circumference-for-age and weight-for-length be obtained at each clinic visit in infants with complex heart disease. These measurements will help determine when a nutritional change or intervention needs to be made. Involvement of a Registered Dietician in the care of at-risk infants and children allows for appropriate monitoring and consultation when interventions are needed.
Standardization of Feeding Practices
Standardization of feeding practices can improve growth and long term outcomes. The NPC-QIC has published recommended feeding algorithms for infants with a single ventricle, with the intent to help centers standardize their nutritional approach to these challenging patients (Figs. 11.2, 11.3, 11.4, and 11.5). Using standardized feeding algorithms have been shown to increase nutrient delivery, decrease length of stay, and minimize morbidities [12, 35–37]. In addition to improving nutritional intake, feeding protocols both before and after cardiac surgery have been shown to reduce the incidence of necrotizing enterocolitis (NEC), minimize interruptions in feeding, improve oral intake, decrease cost and hospital length of stay as well as improve overall outcomes [38, 39]. The use of an interdisciplinary team also has been shown to improve oral intake and overall nutritional management in high-risk neonates and infants [40]. The use of a standardized approach has been successful in infants with hypoplastic left heart syndrome following stage 1 palliation. Braudis et al. demonstrated significantly reduced post-operatively times to reach the recommended daily allowance of calories from 13 days to 9 days in this group of patients [36]. Suboptimal growth in cardiac patients is multifactorial leading to a complex process of feeding and dysfunctional feeding postoperatively. Implementation of care bundles driven by multidisciplinary teams is a strategy that can address not only the medical and physical needs of the infant but also the behavioral and environmental factors that may affect nutrition and growth [38].
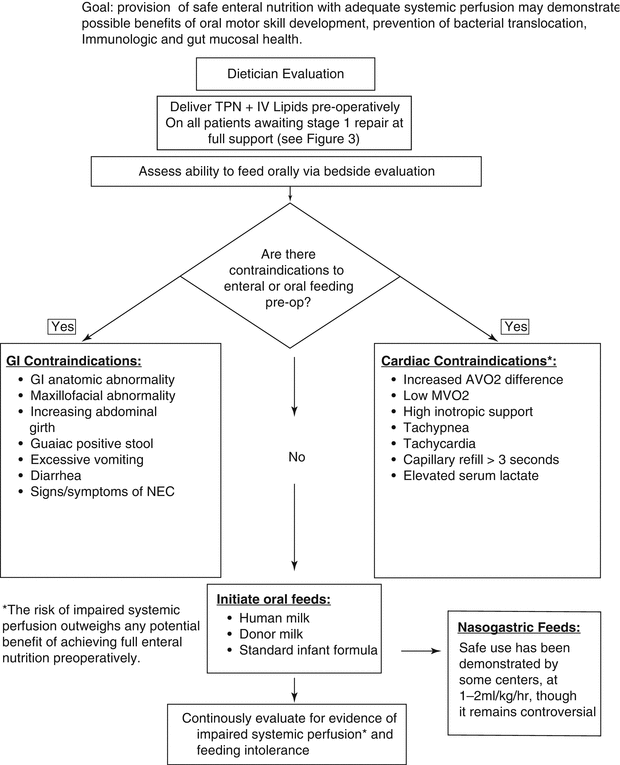
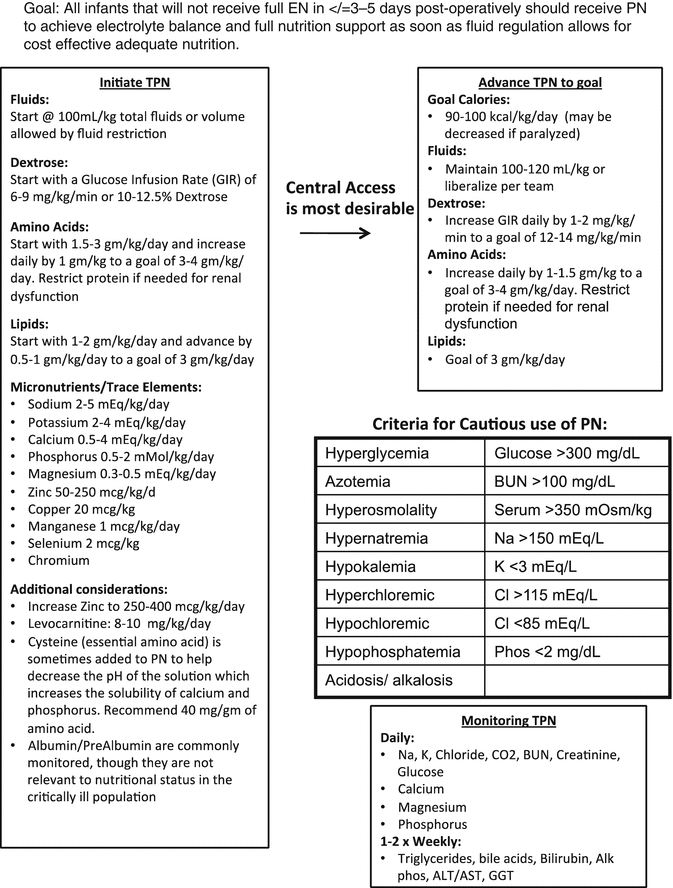
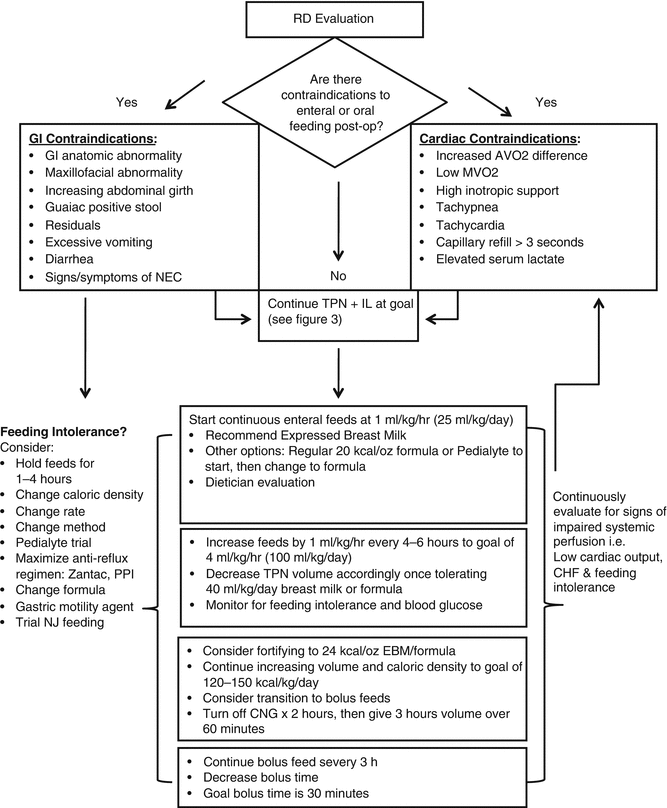
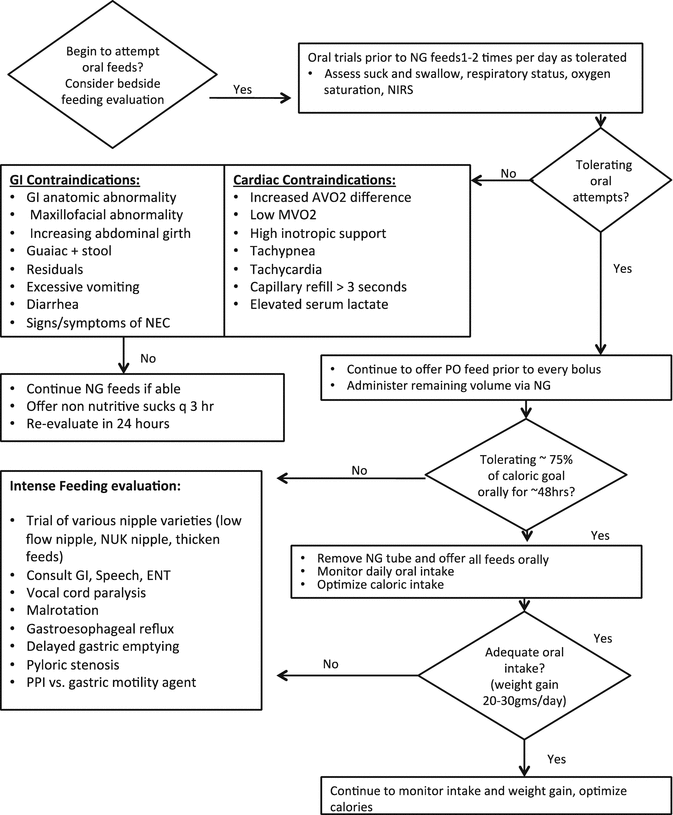

Fig. 11.2
Pre-operative enteral feeding protocol

Fig. 11.3
Post operative Total Parental Nutrition Protocol (TPN)

Fig. 11.4
Post operative Enteral Feeding (EN)

Fig. 11.5
Post operative oral feeding protocol
Preoperative Feeding
In infants undergoing surgical repair or palliation of congenital heart disease there are practices that can be instituted that may help the patients meet nutritional needs and goals. During the preoperative period feeding is helpful to promote normal development of feeding patterns in neonates, prevent translocation of bacteria and to promote immunologic and gut mucosal health. Current evidence suggests that enteral feeding can be attempted with close monitoring and vigilance in patients with hemodynamic stability. In most cases, sick neonates will need supplemental parenteral nutrition to provide adequate nutrition. Postoperatively there should be a goal for early introduction of enteral feeding. Intermittent oral and nasogastric feeding preoperatively has been successful and safe in many studies of infants with congenital heart disease although it has been shown that continuous feeding requires more time to reach the caloric goals [41, 42]. Initiation of enteral nutrition should be started at 20–25 mL/kg/day. Human or Donor milk is the preferred fluid but if not available a standard infant formula is an acceptable alternative. Advancement of feeds should be increased by 20 mL/kg/day to a goal of 120–150 ml/kg/day. Feeding intolerance is an important clinical sign that can suggest poor systemic perfusion in conjunction with poor cardiac output. If an infant cannot feed preoperatively or for a prolonged period of time post-operatively, non-nutritive measures should be provided to continue to develop oral motor skills.
Potential Post-operative Feeding and Nutrition Difficulties
Vocal cord injury is a common post-operative complication following congenital heart repairs involving aortic arch reconstruction, particularly after the Norwood operation in infants with hypoplastic left heart syndrome and due to prolonged intubation [43, 44]. Infants with vocal cord injury often have difficulty feeding and require speech therapy during its resolution [12]. Because of this common finding it is recommended that thorough speech and feeding evaluations be performed following infant aortic arch reconstruction surgery. This highlights the importance of multidisciplinary teams in the care of these complex infants. Speech and language pathologists offer critical assessment skills in the area of feeding and swallowing dysfunction diagnosis and management.
Chylous effusion can be a serious complication following cardiovascular surgery and can significantly impair efforts at feeding in the post-operative period. The incidence of chylous effusion is reported to be from 0.05 to 9 % of infants and children undergoing surgery for congenital heart disease [45]. Potential etiologies of chylothorax include thoracic duct injury, increased right-sided pressures and central vein thrombosis [46–48]. Chylous fluid consists of high levels of triglycerides, lymphocytes, and proteins including immunoglobulins and clotting factors [48]. Given the high nutrient content of the fluid, increased drainage puts patients at risk for protein-energy malnutrition, delayed initiation of enteral nutrition, electrolyte disturbances, coagulopathy, poor wound healing, impaired immune function, and respiratory complications due to required chest tube placement [49, 50].
Potential Adverse Events Secondary to Feeding
When feeding infants with congenital heart disease, even when following conservative guidelines, there are risks of adverse outcomes. The primary of these risks include the development of necrotizing enerocolitis and problems associated with feeding via a nasogatric tube. Infants with impaired systemic perfusion are at risk for necrotizing enterocolitis (NEC) [15]. Mortality in post-operative infants with congenital heart disease is significantly higher in those who develop NEC than those that do not [51]. Feeding intolerance can be the first indication of NEC, especially when associated with bloody stools. Any infant with concerns for NEC in the post-operative period should be fully evaluated with clinical examination, abdominal radiographs and appropriate consultation. Feeds should be immediately held during this evaluation period.
It is common to use a nasogastric tube to supplement feeding in infants with poor oral intake. This form of enteral feeding is used both in and out of the hospital setting [34]. While uncommon, one problem that has been associated with nasogastric tube feedings is misplacement of the tube and aspiration [52]. It is essential to educate caregivers of the potential risks of enteral feeding as well as the signs of problems with the nasogastric tube.
Future Work and Research Questions
While there has been improvement in our understanding of nutritional problems in infants and children with congenital heart disease, there are still aspects of this problem that require additional work. Standardization of feeding practices does improve overall patient growth but there is still a poor understanding of the etiology of growth problems in many forms of congenital heart disease. Several areas that may be addressed include actual energy expenditure, functional gastrointestinal perfusion studies, and biomarkers of nutritional problems. In addition to physiologic causes of poor growth, work should progress to understand the impact of the home environment on growth in these children with complex medical needs.
Finally, while it is important to continue to understand the causes of growth failure it is important for investigators to better understand the long-term problems associated with early struggles with growth, perhaps most importantly the role that early growth plays in neurodevelopment, neuro-recovery and plasticity and long term wellbeing.
Conclusions
Growth failure is well recognized and common in infants with complex congenital heart disease [5–7]. Adequate nutrition is essential for normal growth and development for infants and children. Growth problems in congenital heart disease can lead to both short and long term problems including an adverse effect on surgical outcomes and impairment of neurodevelopment. While the etiologies of growth problems in congenital heart disease are varied and multiple, much progress has been made in understanding these issues and determining interventions that mitigate this important problem. A multi-disciplinary approach to nutritional management and surveillance, and the use of standardized nutritional protocols, with the intention of early identification and mitigation of growth problems can improve growth in infants with congenital heart disease and further improve outcomes for these challenging patients.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


