Bacteria
Viridans group streptococci (alpha-hemolytic)—S. mitis, S. sanguis, S. mutans, etc.
Staphylococcus aureus
Coagulase-negative staphylococcus
Streptococcus pneumonia
Streptococcus bovis and other streptococci
Enterococcus species
HACEK (Hemophilus species, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikinella species, Kingella kingae)
Pseudomonas species
Culture negative (Chlamydia species, Coxiella burnetii, Abiotrophia species, Bartonella species, Brucella species, Legionella species, etc.)
Fungal
Candida albicans
Candida tropicalis
Histoplasma capsulatum
Aspergillus
Cryptococcus neoformans
A number of conditions can predispose to IE, including mitral valve prolapse, rheumatic heart disease, indwelling catheters, intravenous drug abuse, prosthetic valves, and both unrepaired and repaired CHD [1]. The role of congenital cardiac disease as a predisposing factor for IE continues to increase in developed countries; CHD now appears to be the predominant underlying condition for IE in children over the age of 2 years [4]. Certain congenital heart lesions are recognized to have an association with IE, including ventricular septal defects, patent ductus arteriosus, aortic valve abnormalities, and tetralogy of Fallot [5]. Furthermore, increasing numbers of children with IE have had previous palliative or corrective surgery for CHD [4, 6]. However, IE can occur in the absence of any structural heart disease; this is seen in approximately 2–5 % of younger pediatric IE cases (2 months to 15 years of age), and 25–45 % of older IE patients (15–60 years of age) [1, 7].
Because of the variability in clinical presentation, the diagnosis of IE is not always straightforward. To assist in diagnosis, a set of criteria—known as the Duke criteria—were proposed in 1994 as a diagnostic schema for patients with suspected IE [8]. Several refinements were made in 2000, resulting in the modified Duke criteria [9]. These criteria incorporate clinical, laboratory, pathologic and echocardiographic criteria, and stratify patients into three main categories—definite IE, possible IE, and rejected—based upon the presence of major and minor criteria (Tables 16.2 and 16.3). “Definite” IE is defined by the presence of two major criteria, or one major and three minor, or five minor criteria. “Possible” IE is defined by one major and one minor criterion, or three minor criteria. “Rejected” indicates absence of evidence supporting the diagnosis of IE. For diagnosis, microbiologic confirmation (as demonstrated by positive blood cultures) is a principal criterion. However, the Duke criteria clearly recognize and acknowledge the importance of echocardiography (with special mention of TEE) in the diagnosis of IE, as evidenced by the inclusion of echocardiographic findings as a major criterion for diagnosis. Indeed, echocardiography is now considered essential in the diagnostic workup and continuing evaluation of IE [10]. The utility of the Duke criteria have been validated by a number of studies in both the adult and pediatric populations [11–14]. They are now widely accepted guidelines for the evaluation of possible IE, both in adults as well as children [4, 15, 16].
Table 16.2
Modified Duke criteria: definition of terms used for the diagnosis of infective endocarditis (IE)
Major criteria |
Blood culture positive for IE |
Typical microorganisms consistent with IE from two separate blood cultures: |
Viridans streptococci, Streptococcus bovis, HACEK group, Staphylococcus aureus; or |
Community-acquired enterococci, in the absence of a primary focus; or |
Microorganisms consistent with IE from persistently positive blood cultures, defined as follows: |
At least two positive cultures of blood samples drawn >12 h apart; or |
All of three or a majority of ≥4 separate cultures of blood (with first and last sample drawn at least 1 h apart) |
Single positive blood culture for Coxiella burnetii or antiphase I IgG antibody titer >1:800 |
Evidence of endocardial involvement |
Echocardiogram positive for IE (TEE recommended in patients with prosthetic valves, rated at least “possible IE” by clinical criteria, or complicated IE [paravalvular abscess]; TTE as first test in other patients), defined as follows: |
Oscillating intracardiac mass on valve or supporting structures, in the path of regurgitant jets, or on implanted material in the absence of an alternative explanation; or |
Abscess; or |
New partial dehiscence of prosthetic valve |
New valvular regurgitation (worsening or changing of pre-existing murmur not sufficient) |
Minor criteria |
Predisposition, predisposing heart condition or injection drug use |
Fever, temperature >38 °C |
Vascular phenomena, major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhage, conjunctival hemorrhages, and Janeway’s lesions |
Immunologic phenomena: glomerulonephritis, Osler’s nodes, Roth’s spots, and rheumatoid factor |
Microbiological evidence: positive blood culture but does not meet a major criterion as noted abovea or serological evidence of active infection with organism consistent with IE |
Echocardiographic minor criteria eliminated |
Table 16.3
Definition of endocarditis from modified Duke criteria
Definite infective endocarditis |
Pathologic criteria |
1. Microorganisms demonstrated by culture or histologic examination of a vegetation, a vegetation that has embolized, or an intracardiac abscess specimen; or |
2. Pathologic lesions; vegetation or intracardiac abscess confirmed by histologic examination showing active endocarditis |
Clinical criteria a |
1. Two major criteria; or |
2. One major criterion and three minor criteria; or |
3. Five minor criteria |
Possible infective endocarditis |
1. One major criterion and one minor criterion; or |
2. Three minor criteria |
Rejected |
1. Firm alternate diagnosis explaining evidence of infective endocarditis; or |
2. Resolution of infective endocarditis syndrome with antibiotic therapy for ≤4 days; or |
3. No pathologic evidence of infective endocarditis at surgery or autopsy, with antibiotic therapy for ≤4 days; or |
4. Does not meet criteria for possible infective endocarditis, as above |
The full diagnostic evaluation and management of IE encompasses a wide variety of aspects, including microbiology, antimicrobial therapy/duration, ongoing assessment, extracardiac complications, and indications and timing of surgical management [1, 17]. Therapy of IE is aimed at (a) eradication of the infecting microorganism; (b) treatment of cardiac complications; (c) prevention and/or treatment of extracardiac (particularly embolic) complications [1]. Antimicrobial therapy (selection/duration) is based upon the microbiology and susceptibility of the causative organism, and continues to evolve as new therapeutic agents are introduced. The role of surgery for IE also continues to evolve, and it is clear that this role has expanded; a recent European survey revealed that >50 % of adult patients with IE underwent surgery [18]. Controversy still exists as to the exact indications and timing for surgery; in most instances surgery is performed for left sided endocarditis, severe valvular involvement, and/or evidence of embolic phenomena [19]. Recently, there has also been published evidence that early surgery for severe left sided endocarditis in adults results in significantly better outcomes than conventional medical therapy [20]. Proposed indications and timing of surgery in adults are listed in Tables 16.4 and 16.5 [19]. In children, the indications for surgery are not as straightforward; surgery tends to be performed less frequently because 80–90 % of children with IE are expected to survive solely with conservative medical treatment [21, 22]. Nonetheless several published series of IE in children have reported an important percentage (between 16–67 %) of cases that required surgical intervention—in patients with and without CHD. In most published reports, the indications for cardiac surgery in pediatric IE patients tend to be similar to those of adults—valve dysfunction, congestive heart failure, septic embolization, and large vegetations. In addition, surgery is performed in CHD patients to repair the underlying cardiac defect (if still present), and also to remove or replace infected prosthetic material such as homografts and aortopulmonary shunts [7, 18, 21, 23–25].
Table 16.4
Indications for surgery in IE
Congestive heart failure a |
Congestive heart failure caused by severe aortic or mitral regurgitation or, more rarely, by valve obstruction caused by vegetations |
Severe acute aortic or mitral regurgitation with echocardiographic signs of elevated left ventricular end-diastolic pressure or significant pulmonary hypertension |
Congestive heart failure as a result of prosthetic dehiscence or obstruction |
Periannular extension |
Most patients with abscess formation or fistulous tract formation |
Systemic embolism b |
Recurrent emboli despite appropriate antibiotic therapy |
Large vegetations (>10 mm) after one or more clinical or silent embolic events after initiation of antibiotic therapy |
Large vegetations and other predictors of a complicated course |
Very large vegetations (>15 mm) without embolic complications, especially if valve-sparing surgery is likely (remains controversial) |
Cerebrovascular complications c |
Silent neurological complication or transient ischemic attack and other surgical indications |
Ischemic stroke and other surgical indications, provided that cerebral hemorrhage has been excluded and neurological complications are not severe (e.g., coma) |
Persistent sepsis |
Fever or positive blood cultures persisting for >5–7 days despite an appropriate antibiotic regimen, assuming that vegetations or other lesions requiring surgery persist and that extracardiac sources of sepsis have been excluded |
Relapsing IE, especially when caused by organisms other than sensitive streptococci or in patients with prosthetic valves |
Difficult organisms |
S aureus IE involving a prosthetic valve and most cases involving a left-sided native valve |
IE caused by other aggressive organisms (Brucella, Staphylococcus lugdunensis) |
IE caused by multiresistant organisms (e.g., methicillin-resistant staphylococcus aureus or vancomycin-resistant enterococci) and rare infections caused by Gram-negative bacteria |
Pseudomonas aeruginosa or Fungal IE |
Q fever IE and other relative indications for intervention |
Prosthetic valve endocarditis |
Virtually all cases of early prosthetic valve endocarditis |
Virtually all cases of prosthetic valve endocarditis caused by staphylococcus aureus |
Late prosthetic valve endocarditis with heart failure caused by prosthetic dehiscence or obstruction, or other indications for surgery |
Table 16.5
Timing of Surgery
Emergency surgery (within 24 h) |
Native (aortic or mitral) or prosthetic valve endocarditis and severe congestive heart failure or cardiogenic shock caused by: |
Acute valvular regurgitation |
Severe prosthetic dysfunction (dehiscence or obstruction) Fistula into a cardiac chamber or the pericardial space |
Urgent surgery (within days) |
Native valve endocarditis with persisting congestive heart failure, signs of poor hemodynamic tolerance, or abscess |
Prosthetic valve endocarditis with persisting congestive heart failure, signs of poor hemodynamic tolerance, or abscess |
Prosthetic valve endocarditis caused by staphylococci or Gram-negative organisms |
Large vegetation (>10 mm) with an embolic event |
Large vegetation (>10 mm) with other predictors of a complicated course |
Very large vegetation (>15 mm), especially if conservative surgery is available |
Large abscess and/or periannular involvement with uncontrolled infection |
Early elective surgery (during the in-hospital stay) |
Severe aortic or mitral regurgitation with congestive heart failure and good response to medical therapy |
Prosthetic valve endocarditis with valvular dehiscence or congestive heart failure and good response to medical therapy |
Presence of abscess or periannular extension |
Persisting infection when extracardiac focus has been excluded |
Fungal or other infections resistant to medical cure |
Despite advances in medical and surgical management, IE remains a serious medical condition, with important risks of morbidity and mortality. A thorough and complete review of this multifaceted topic, including diagnosis, antimicrobial considerations, and medical/surgical therapy, would require its own separate chapter. For the purposes of this chapter, it is important to understand the integral role that echocardiography—and particularly TEE—plays in the many different aspects of the diagnosis and management of IE. This will be discussed below.
Echocardiographic Manifestations of IE
There are several ways in which IE can appear by echocardiography. The principal echocardiographic manifestations of IE include the following.
Vegetations
Vegetations are the most characteristic finding associated with endocarditis. They represent a mass of pathologic organisms nestled within a weave of platelets, red blood cells, and fibrin. Vegetations often develop in an area where the endothelium has been injured or disrupted by an abnormal high velocity jet or intravenous catheter; this usually occurs on a valvar surface, but can also be seen on a cardiac chamber wall when the endothelial surface has been injured. Vegetations also have the propensity to develop on foreign material such as a prosthetic valve or patch. The surface of the damaged endothelium or prosthetic material serves as a nidus for platelet/fibrin deposition, producing a thrombus (vegetation) at the site. This vegetation is at first sterile, but with bacteremia, circulating microorganisms can become adherent to the meshwork, resulting in an infected vegetation. Infection triggers further deposition of platelets and fibrin over the microorganisms; the organisms embedded within the vegetation are then shielded from host defense mechanisms, allowing them to proliferate rapidly and produce further growth of the vegetation [26]. Vegetations can have a number of detrimental effects: (a) they can grow and destroy adjacent tissue; (b) organisms can be released continuously into the bloodstream, leading to persistent bacteremia and hematogenous seeding of remote sites; (c) pieces of the vegetation can break off and embolize to other organs (brain, lung, kidney), sometimes producing serious and even devastating complications; (d) antibody response to the infecting organisms leads to subsequent tissue injury by immune complex deposition [1].
By echocardiography, vegetations are echogenic masses, generally irregular in shape and variable in size. They can localize anywhere on an affected valve or nonvalvar structure, though they tend to arise on a valve or endothelial surface “downstream” to a high velocity jet, e.g. adjacent to a ventricular septal defect or valvar regurgitant jet. They are usually freely mobile, oscillating with the cardiac cycle, and can move back and forth within the plane of a valve. Intracardiac vegetations are generally very well seen by TEE (Fig. 16.1, Video 16.1).
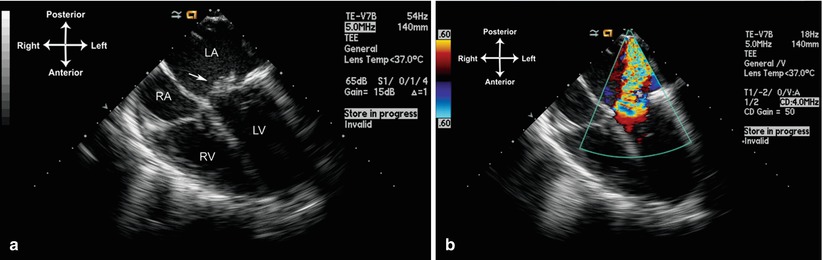

Fig. 16.1
Large vegetation (arrow) on the anterior leaflet of mitral valve (a), which resulted in chordal destruction and severe mitral regurgitation (b). Mid esophageal view four chamber (multiplane angle 0°). LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Valvar Dysfunction
The echocardiographic manifestations of valvar dysfunction include ruptured chordae with prolapsing or flail leaflets, fenestrations in the valve cusps, and torn leaflets. All of these lead to disruption of valvar function and resultant valvar regurgitation, often to a significant degree. The amount of regurgitation, and the area of origin, can be well-seen using color flow Doppler. It is not uncommon to see vegetations in association with valvar disruption, as evidence of the destructive process from IE. Such patients not only appear toxic from their infection, but might also suffer symptoms of congestive heart failure if valvar incompetence is significant. Examples of valve disruption and accompanying vegetation are shown in Figs. 16.1 and 16.2, Videos 16.1 and 16.2.
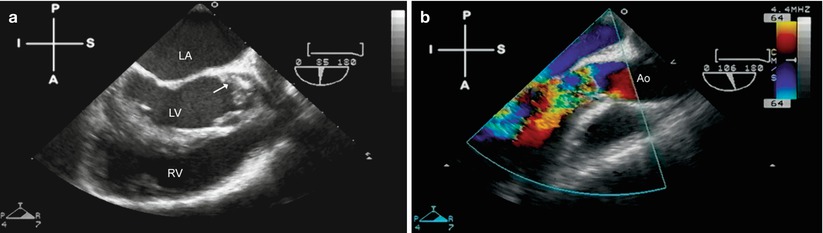

Fig. 16.2
Aortic valve endocarditis, seen from a mid esophageal aortic valve long axis view (multiplane angle 85°–106°). Figure a shows a prominent vegetation (arrow) on the left coronary cusp, which has caused significant cusp destruction and resulted in severe aortic valve regurgitation (b). Ao ascending aorta, LA left atrium, LV left ventricle, RV right ventricle
Intracardiac Abscesses
Intracardiac abscess formation results from suppurative extension of the infective process. Purulent cavities form as the infection spreads into adjacent tissue. Most commonly, this occurs with native aortic valve IE, as infection extends into the weakest portion of the annulus—the membranous septum and atrioventricular node. When this occurs, heart block is a frequent sequela. Perivalvar abscess formation occurs in 10–40 % of all native IE; as noted, it is most common in native aortic valve IE, less common with native tricuspid or mitral IE [10]. Perivalvar abscesses are seen even more frequently with prosthetic valve IE, occurring in 56–100 % of patients [10, 27]. The characteristic echocardiographic appearance of abscess formation is as an echo-free space, representing purulent fluid, either within the wall surrounding the affected valve (e.g. the aortic root in aortic valve endocarditis), or extending into the adjacent tissue. In those patients with an abscess surrounding a prosthetic mitral valve, valve dehiscence is often seen. For the evaluation of abscesses, TEE has been shown to improve sensitivity dramatically compared to transthoracic echocardiography (TTE), and it is the preferred modality for diagnosis of perivalvar abscesses [28]. An example of an abscess that formed around a prosthetic aortic valve is shown in Fig. 16.3, Video 16.3.
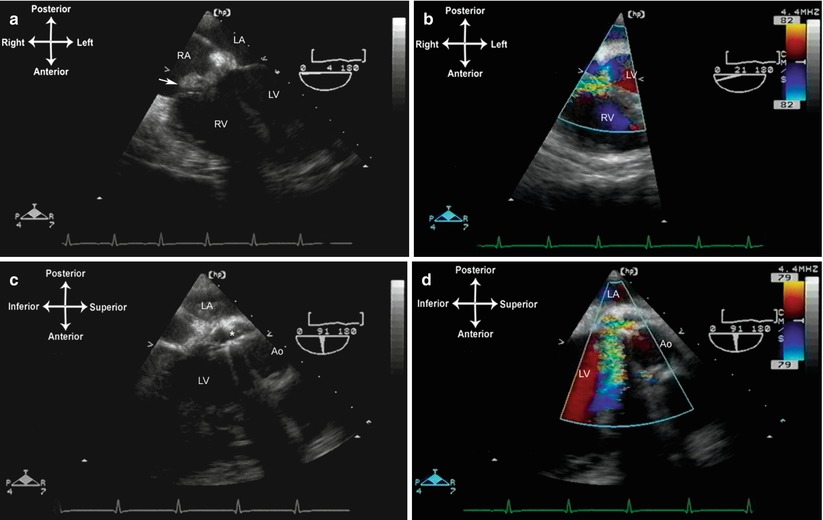

Fig. 16.3
Endocarditis in a patient with a prosthetic aortic valve (St. Jude bileaflet tilting disk valve). (a, b). The mid esophageal four chamber view demonstrates a perivalvar abscess that extends into the noncoronary cusp, causing a fistulous tract communicating with the right atrium. A large vegetation (arrow) has developed in this area and shunting is seen into the right atrium. (c, d). Mid esophageal aortic valve long axis view, angle about 90°. There is marked aortic regurgitation seen through an area of valve dehiscence . Ao aorta, LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Aneurysm Formation/Fistulous Tracts
If there is extension of the infection to the adjacent vessel wall, destruction of wall tissue can occur, leading to thinning and aneurysm formation. This can be seen especially with aortic valve IE, in which fistulous tracts can form between the sinus of Valsalva and an adjacent cardiac structure (i.e. an infected sinus of Valsalva aneurysm), or a fistulous tract can form into the pericardial space [29, 30]. In this setting, TEE will demonstrate the aneurysmal dilation of the vessel wall, and color flow Doppler will show systolic/diastolic (or continuous) flow between the aorta and the receiving chamber (Figs. 16.3 and 16.4, Videos 16.3 and 16.4). Infective endarteritis and pseudoaneurysm formation can also occur, particularly in areas of foreign material such as suture lines and biologic grafts [31, 32] (Fig. 16.5, Video 16.5).
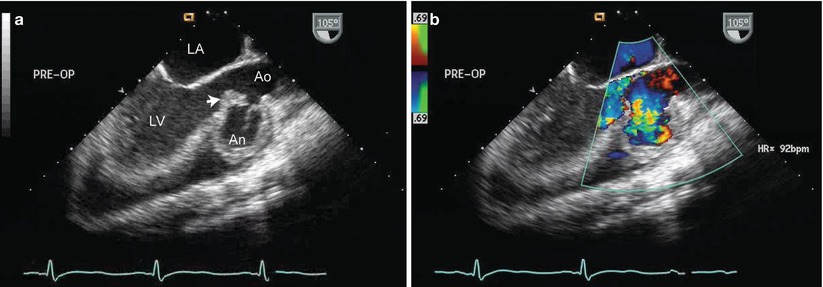
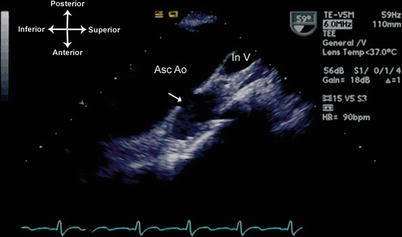

Fig. 16.4
Infected sinus of Valsalva aneurysm from aortic valve endocarditis, obtained from the mid esophageal aortic valve long axis view. Figure a shows a large vegetation of the aortic valve (arrow) and erosion of the right sinus of Valsalva, producing a large aneurysm (An). Figure b shows blood filling the aneurysm during diastole. Ao ascending aorta, LA left ventricle, LV left ventricle

Fig. 16.5
Infected pseudoaneurysm off ascending aorta. This TEE was performed to evaluate the aortic valve in a patient with a previous aortic valve surgery and persistent fungemia. A large pseudoaneurysm (arrow) was discovered using an upper esophageal view, multiplane angle 60°. At surgery, the pseudoaneurysm was found to be infected and filled with fungus. Note that the superior portion of aorta and innominate vein can be seen well in this patient by TEE. Asc Ao ascending aorta, In V innominate vein
Congestive Heart Failure/Pericardial Effusion
Congestive heart failure (CHF) is a known complication of IE, and one associated with a poor prognosis [33–35]. It can occur as a result of several different complicating processes associated with IE: native valve destruction/perforation/chordal rupture, prosthetic valve dehiscence, abscess formation causing heart block, sudden intracardiac shunts due to fistulous tracts, and septic emboli to the coronaries causing myocardial ischemia/infarction [10, 19]. In addition, myocardial dysfunction can be seen with IE, either from myocardial toxicity due to the IE process (e.g. large abscess), ventricular decompensation from valvar regurgitation, or the overall septic process causing generalized depression of myocardial function (particularly with a virulent organism such as Staphylococcus aureus). In native valve IE, acute CHF is more frequently seen with left sided infections—aortic (29 %) and mitral (20 %)—than with tricuspid infections (8 %) [10]. A pericardial effusion can also be seen in patients with IE; it can be infectious, resulting from hematogenous seeding of the pericardium or as a direct extension from intracardiac IE (e.g. perforation of a perivalvar abscess). Rarely, it can occur as a reactive/serous effusion [36].
Use of TEE for Evaluation of Infective Endocarditis
As noted above, echocardiography is considered essential in the diagnosis and management of IE. The major question is whether to perform TTE or use TEE to evaluate for vegetations. In adults, a number of studies have confirmed the superior diagnostic sensitivity of TEE over TTE for IE [37–40]. This is particularly true in those patients with intracardiac abscesses and prosthetic valve endocarditis, in whom TEE appears clearly superior for diagnosis [28, 41]. In children, the advantages of TEE over TTE are less apparent, particularly with the infant and younger child, in whom TTE generally provides excellent imaging. For younger pediatric patients, TTE is usually adequate for the diagnostic evaluation of IE [42]. In most pediatric patients, particularly the younger age groups, the evidence suggests that TEE can be reserved for those patients in whom imaging quality is felt to be suboptimal. A recent study in pediatric patients ages 10 days to 17.5 years (over half of whom had CHD) showed that TTE, when used in conjunction with the Duke criteria, was useful for the diagnosis of IE; the authors recommended TEE only for an “inadequate” TTE study or when there was an organism with a high association of aortic root abscess [43].
The evaluation of IE by TEE can occur in several settings. It can be performed in the ICU or ambulatory setting, serving as either diagnostic evaluation for suspected IE or as a monitoring procedure for a patient receiving treatment for known IE. It also plays a vital role in the operating room. Preoperatively, TEE is used to assess not only the vegetation or abscess, but also valvar function and the surrounding cardiac structures. When applicable, prosthetic valve dehiscence and pseudoaneurysm formation can also be evaluated. Postoperatively, TEE is used to assess the results of operative repair or valve replacement, and to guide perioperative hemodynamic management [19, 36].
If TEE is performed to evaluate for IE, a complete study should be performed using all major TEE views/windows, as outlined in Chap. 4. Assessment should focus on a number of details. If a vegetation is present, its appearance and motion should be evaluated in multiple planes, and linear measurements can be obtained. The risk of embolic events appears to be greatest with vegetations >10 mm on the anterior leaflet of mitral valve [44]. Valve leaflet anatomy and motion should also be evaluated both by imaging and color flow Doppler, with attention paid to any valvar perforation or chordal disruption. In the case of aortic valve endocarditis, a careful evaluation should also be made for possible abscess or aneurysm formation. If a prosthetic valve is in place, a thorough assessment should be performed for vegetations, valve leaflet motion, and possible perivalvar abscess/dehiscence. Color flow Doppler is useful to determine valve competency and flow profile. A complete evaluation should be performed of other cardiac structures to rule out structural defects and/or other potential sites of IE, including the aorta and pulmonary artery (when the pulmonary artery can be visualized by TEE). Rarely, cases of endarteritis can also be found by TEE (Fig. 16.5, Video 16.5). Finally, myocardial function should also be assessed.
Three caveats are important to consider. First, even with TEE, not all vegetations will be visible, particularly if the vegetations are smaller than the resolution limits of the TEE probe and/or TEE imaging is suboptimal. This is an important consideration in patients with operated and unoperated CHD, who can have vegetations located in areas not readily visible by TEE (e.g. a Blalock-Taussig shunt). Studies have shown that—irrespective of whether TTE or TEE is used—patients with CHD and IE are less likely to have visible vegetations [5]. Thus, the echocardiographic data should be considered in the context of the entire clinical picture, as noted with the Duke criteria listed above. In some cases, if IE is still suspected, a TTE or TEE can be performed 7–10 days later to determine if a vegetation or abscess has appeared [10]. The second important caveat is that not all echogenic masses represent vegetations. Sterile thrombi, tumors, irregular valve excrescences, and foreign material (such as suture material) can sometimes resemble vegetations. Again, the echocardiogram should be reviewed in conjunction with the entire clinical picture. If previous echocardiograms are available (either transthoracic or transesophageal), these can be very useful to make direct comparisons to determine whether an abnormal finding is new or longstanding. New findings are much more suspicious for IE. The last important caveat is that not all vegetations are infectious. A number of medical conditions can produce sterile vegetations adherent to valvar surfaces. Examples of these include systemic lupus erythematosus (Libman-Sachs endocarditis), and nonbacterial thrombotic endocarditis (NBTE, also known as marantic endocarditis). The latter can occur as complication of malignancy, uremia, burns, hypercoagulable states, or autoimmune diseases, and it has been found in approximately 1.2 % of all autopsy patients, although the reported incidence is between 0.3–9.3 % [1, 45]. In fact, Libman-Sachs endocarditis is felt to be a form of NBTE [46]. These vegetations are usually seen on the valve closure contact line of the atrial surface of the atrioventricular (AV) valves and ventricular surface of the semilunar valves. In many cases, the vegetations are benign and clinically inapparent. However, systemic embolization has been described in up to 30–50 % of patients [45–47], with a tendency towards embolization to the brain, kidney, spleen, mesenteric bed, or extremities [46, 48].
Cardiac Masses
Aside from infective endocarditis, the most common pathologic cardiac masses are thrombus or tumors. The role of TEE in the diagnostic assessment of cardiac masses is generally complementary to TTE, particularly in pediatric patients. In most cases, TTE is more than adequate to evaluate the location and extent of a cardiac mass. However in older patients, and in those with poor echocardiographic windows, TEE might be called upon to assist in evaluation. For instance, TEE has become well established in adults as a requisite tool for evaluation of the etiology of ischemic strokes; approximately 15–20 % are caused by cardiogenic emboli (thrombi, tumors, vegetations, etc.) [49].
Cardiac Thrombi
Thrombi can vary considerably in their echocardiographic appearance and location. They are generally echogenic, relatively homogeneous in density, and irregular in shape. In some instances there can be calcification within the thrombus. They are generally attached to some structure in the heart, whether it is an endocardial surface, AV valve, or some type of foreign material. The attachment point is usually broad-based, but it can also be thin and pedunculated in appearance. In most clinical settings, thrombi are associated with an indwelling catheter, although in certain pathologic situations—e.g. where there is stasis of blood—they can arise spontaneously. An important example of this is dilated cardiomyopathy, in which reduced cardiac motion leads to stasis of blood flow and a propensity for thrombus formation, particularly near the apex of the left ventricle (Fig. 16.6, Video 16.6). In those situations in which a catheter is present, usually the superior vena cava (SVC) or right atrium, TEE can be used to evaluate the attachment point, size, and extent of the thrombus. Mid to upper esophageal views, with the probe rotated rightward and multiplane angle 80–95° (mid esophageal bicaval view), permit a sagittal visualization of the length of the SVC as it returns to the right atrium (Fig. 16.7, Video 16.7). If an indwelling catheter is present, it can be visualized and evaluated for a possible attached thrombus. It is important not to misinterpret normal structures or variants, such as the crista terminalis (the muscular ridge in the interior of the right atrium separating right atrial appendage from the remainder of the atrium), as an intracardiac thrombus. Color flow Doppler can evaluate the flow returning through the SVC to the right atrium. If there is obstruction to SVC return, a mean gradient can sometimes be obtained using the deep transgastric views (long axis and sagittal), rightward turning of the probe, and anteflexion to achieve posterior angulation (see Chap. 4). These maneuvers enable visualization of SVC flow as it enters the right atrium, and also provide an excellent angle of insonation for spectral Doppler interrogation.
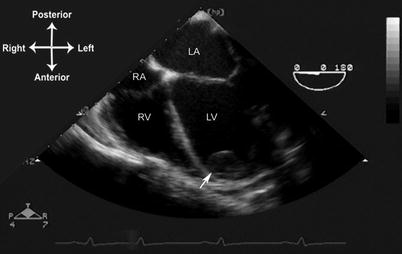
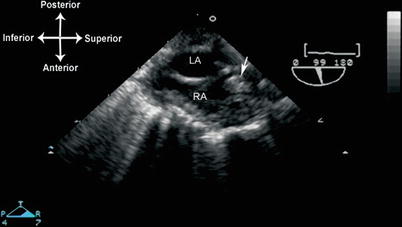

Fig. 16.6
Thrombus (arrow) in the left ventricular apex of a patient with Duchenne muscular dystrophy and dilated cardiomyopathy. Mid esophageal four chamber view, multiplane angle 0°. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle

Fig. 16.7
Thrombus (arrow) in the superior vena cava, probably associated with a catheter. Seen from mid esophageal bicaval view, (multiplane angle 99°). LA left atrium, RA right atrium
Thrombi sometimes develop in the left (and less frequently, the right) atrial appendage in patients, particularly adults with severe mitral stenosis and/or atrial fibrillation [50, 51]. In adult studies, the incidence of left atrial thrombus in patients with atrial fibrillation ranges between 10–15 %, and right atrial thrombus 0.4–7.5 % [52]. These thrombi are often challenging to visualize by TTE [53]. It can also be difficult to distinguish these thrombi from pectinate muscles. In this setting, TEE proves invaluable because of the close proximity of the esophagus to both atria, providing excellent visualization of all atrial structures, including the atrial appendages [54, 55]. The left atrial appendage can be well visualized by TEE using the mid esophageal views and leftward rotation, with slight anteflexion to bring the appendage into view (Fig. 16.8, Video 16.8). Rotating the multiplane angle between 0° and 90° affords different views of the structure. The right atrial appendage is best seen from the mid esophageal bicaval view, multiplane angle 85–90°; it is seen anterior to the SVC/right atrial junction. The multiplane angle can also be rotated to 0° to visualize the right atrial appendage from a different plane. Again, it is important to inspect carefully both the right and left atrial appendages, and to distinguish pectinate muscles and multiple appendage lobations [56] from actual thrombus [54, 57].
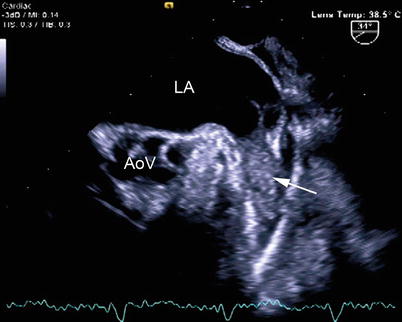

Fig. 16.8
Thrombus in the left atrial appendage (arrow), as viewed from a modified mid esophageal aortic valve short axis view with leftward rotation. There are mobile filamentous strands arising from the thrombus. AoV aortic valve, LA left atrium (Photograph courtesy of Siemens Medical Systems USA, Inc. © 2012–13 Siemens Medical Solutions USA, Inc. All Rights Reserved)
There are certain normal anatomic structures that can resemble a thrombus, and must therefore be inspected carefully by TEE. As previously noted, the crista terminalis can occasionally be mistaken as a thrombus. A Chiari network can sometimes be confused for a thin thrombus or vegetation. This structure, an embryologic remnant of the right valve of the sinus venosus, is usually very mobile and can exhibit considerable anatomic variation [58]. It is attached to the Eustachian and Thebesian valves; it is thin and redundant and often displays a porous, fenestrated or filamentous appearance. A related structure, a prominent Eustachian valve, can be very prominent and extend into the right atrium. It can also be very mobile, and in some cases has the appearance of long vegetation or thrombus. Both a Chiari network and Eustachian valve are well seen using the mid esophageal bicaval view, with multiplane angle approximately 80–90°. In the left atrium, a prominent ridge between left upper pulmonary vein and left atrial appendage (sometimes informally called the “coumadin ridge”) can, on occasion, be mistaken for a thrombus. Another important artifact that may resemble an intracardiac thrombus is the presence of spontaneous echo contrast, or “smoke”, in an atrial chamber [50, 59, 60]. This represents red cell aggregation from sluggish blood flow, such as that seen in a low-flow Fontan circuit (Chap. 10). When dense and echogenic, it can produce the appearance of thrombus. However, the swirling and circular movements differentiate this type of finding from a thrombus (Fig. 16.9, Video 16.9).
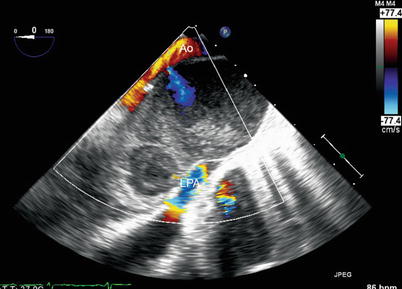

Fig. 16.9
Example of spontaneous echo contrast in a patient after repair of D-transposition of the great arteries, with a pseudoaneurysm arising from a previous cannulation site in the aorta. Image obtained from the upper esophageal aortic arch long axis view. Note the visible swirling of flow due to red cell aggregation. The left pulmonary artery (LPA) is compressed by the pseudoaneurysm. Ao ascending aorta
Cardiac Tumors
Cardiac tumors are divided into two categories: primary and secondary/metastatic. Primary cardiac tumors originate in the heart, and most of these are benign [61–64]. However despite their “benign” histopathology, some primary tumors can still cause significant clinical sequelae due to arrhythmias, mass effect (obstruction), or embolization. Rarely, primary cardiac tumors are malignant and, if so, they are usually sarcomas and are almost always associated with a poor outcome [62]. The other class of cardiac tumors comprises the secondary/metastatic tumors that are, by definition, malignant. Secondary cardiac tumors occur mostly as distant metastases, but they can also invade the heart by direct extension, for example via the inferior vena cava (IVC). Secondary malignant cardiac tumors are seen much more frequently than primary cardiac tumors, both in adults and children. Indeed, in the adult literature metastatic tumors outnumber primary cardiac tumors by a 20–30 to 1 ratio, and in children, by a 3–4 to 1 ratio [61, 62]. In adults, the most common primary sites for metastasis to the heart include lung, breast, lymphoma/leukemia, esophageal, uterine and melanoma. Most of these metastases are to the pericardium, and 90 % are clinically silent [63]. In children, the most common tumors with metastasis to the heart are non-Hodgkin’s lymphoma, neuroblastoma, Wilms tumor, and soft tissue/bone sarcomas [61]. Direct extension of a tumor can occur from the IVC to the right atrium in patients with Wilms tumor [65], renal myosarcoma, or adrenal and hepatocellular carcinoma (Fig. 16.10, Video 16.10). As with adults, overt clinical signs and symptoms of secondary tumors are uncommon [61].
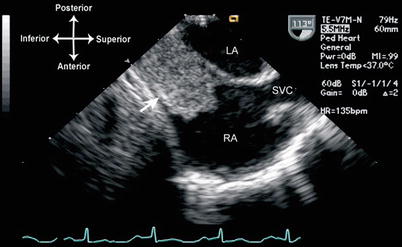

Fig. 16.10
Wilms tumor (arrow) invading the right atrium by direct extension from the inferior vena cava, as seen from the mid esophageal bicaval view, multiplane angle 113°. LA left atrium, RA right atrium, SVC superior vena cava
The echocardiographic presentation of cardiac tumors is highly variable, depending upon the type of tumor and the site of involvement. Cardiac tumors can involve only endocardium, only myocardium, only pericardium, or various combinations thereof [62]. There can be one or multiple foci, again depending on tumor type. An extensive discussion of cardiac tumors is beyond the scope of this chapter, and there are other excellent references available on the subject [62, 63, 66]. The remainder of this section will discuss a few of the more common primary cardiac tumors and provide examples of their evaluation by TEE. The method of TEE evaluation for these particular tumors can be applied to the evaluation of cardiac tumors of any etiology.
Rhabdomyoma
Rhabdomyomas are the most commonly encountered primary cardiac tumors in the pediatric age group, comprising approximately 60–80 % [67, 68]. Typically, multiple tumors are located within the free wall of the right and left ventricle, as well as the interventricular septum. They can also be present in the right atrium. There is a strong association (at least 50 %) with tuberous sclerosis. These tumors are histologically benign but nevertheless patients can be clinically symptomatic in a number of different ways. Depending upon location, atrial or ventricular arrhythmias can occur. Tumors of sufficient size adjacent to an area of inflow or outflow can cause significant obstruction, which occasionally necessitates surgical resection. However with time, spontaneous regression of these tumors usually occurs, so surgery is not indicated in most patients unless the tumors cause significant mass effect.
Given the generally benign nature of cardiac rhabdomyomas, and the young age that these tumors are first diagnosed, it is very rare that a TEE is necessary for evaluation; TTE generally suffices. The major indication for TEE is in patients undergoing surgery for relief of inflow or outflow tract obstruction. Rhabdomyomas are characteristically homogenous, echogenic, and well circumscribed; they are easily distinguished from the surrounding myocardium. The mid esophageal four chamber (ME 4 Ch) view (multiplane angle 0°) is the best view from which to begin evaluation of the tumors, both ventricular and (if present) atrial. Once located, they can be visualized more closely with variations in multiplane angle, along with anteflexion and retroflexion to bring the tumor(s) into view. If a large tumor is causing possible obstruction to AV inflow, color flow and spectral Doppler should be used to evaluate for disturbed flow patterns around the tumor, and to determine a mean gradient across the area. If the tumor is causing possible outflow tract obstruction, mid esophageal views such as the ME 4 Ch, mid esophageal long axis (ME LAX), and mid esophageal right ventricular inflow-outflow (ME RV In-Out) with multiplane angles varying between 0° to 110° are best to evaluate the anatomic extent of the tumor and any turbulence as seen by color flow Doppler (Fig. 16.11, Video 16.11). Deep transgastric long axis (DTG LAX) and deep transgastric sagittal (DTG Sagittal) views with probe anteflexion/retroflexion provide the best angle of spectral Doppler interrogation of the ventricular outflow tracts. The transgastric views also provide other perspectives from which to visualize both right and left sided tumors. These views include the transgastric basal short axis (TG Basal SAX), mid short axis (TG Mid SAX), two chamber (TG 2 Ch), long axis (TG LAX), and right ventricular inflow (TG RV In).
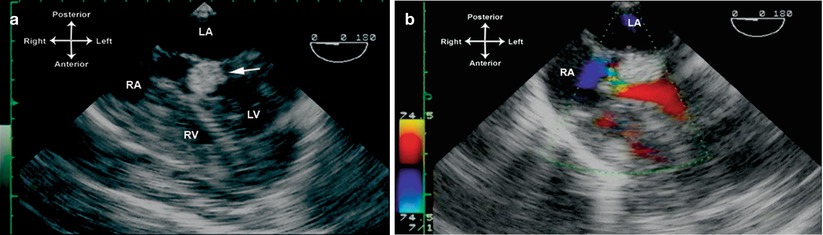

Fig. 16.11
Multiple rhabdomyomas in a patient with tuberous sclerosis, including one that caused near complete obstruction of the left ventricular outflow tract. Figure a is obtained from a mid esophageal four chamber view, multiplane angle 0°, showing a large tumor in the outflow tract (arrow). Turbulent color flow Doppler is seen in (b). This tumor was resected surgically. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Fibroma
Fibromas are the second most common cardiac tumor encountered in the pediatric age group (10–30 %) [69]. They are generally solitary and intramural, located within the ventricular septum or left ventricular free wall [62]. When large, they can impinge significantly upon the adjacent cardiac chamber (usually the left ventricle), causing symptomatology such as congestive heart failure and cyanosis. Rarely, they can be multiple and involve other parts of the heart including the ventricular conduction system, right ventricle and right ventricular free wall. They frequently cause ventricular arrhythmias [70]. Because these tumors do not regress spontaneously and sometimes can grow significantly, surgical resection is recommended.
The TEE evaluation of fibromas generally occurs in the operating room setting, and is similar to that involved with the evaluation of other cardiac tumors. The tumors are best seen using the mid esophageal views, with a combination of multiplane angles between 0° and 100°. Color flow and spectral Doppler should be employed to evaluate for potential compromise of ventricular inflow or outflow due to the tumor. The echocardiographic appearance of a fibroma is that of a single, bright, echo-dense intramural mass with calcifications and cystic areas within the tumor (Fig. 16.12, Video 16.12).
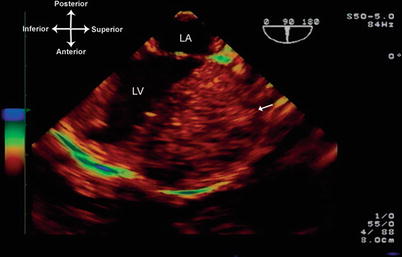

Fig. 16.12
Fibroma attached to the left ventricular free wall, visualized from mid esophageal long axis view, multiplane angle 90°. The fibroma (arrow) is very large, circumscribed, and has a heterogeneous appearance, studded with echolucent areas most likely representing cystic degeneration or necrosis. LA left atrium, LV left ventricle
Myxoma
Myxomas represent the most common primary cardiac tumor in adults [62, 71]. On occasion, they can also be seen in children. They are most commonly located in the left atrial cavity (75–90 %), but can also be found in the right atrium [72], as well as the ventricles [62, 73]. Rarely, they can be present in more than one cavity [74]. Clinical manifestations of a left atrial myxoma are quite variable and can include embolic phenomena and/or obstruction to blood flow causing breathlessness, syncope, and congestive heart failure. Other constitutional symptoms such as fever, malaise, weight loss, and myalgias/arthralgias can also be present. Familial occurrence is reported in approximately 7–10 % of all myxomas, generally in younger patients. These are associated with multiple endocrine syndromes including LAMB (lentigines, atrial myxoma, mucocutaneous myxoma, and blue nevi) and NAME (nevi, atrial myxoma, neurofibromata, and ephelides).
Myxomas are gelatinous in consistency and they can be pedunculated. By echocardiography, they have a globular, lobulated, or fimbriated appearance, and a pedicle can be present. There may be small lucencies and calcifications in the tumor, resulting in an inhomogeneous appearance. Left atrial myxomas characteristically have attachments to the atrial septum, but they can also attach to other portions of the left atrium or to the mitral valve. If large enough, the myxoma can cause obstruction to mitral inflow. Given the numerous potential complications, including embolus, the treatment for atrial myxomas is surgery, and TEE plays an important role in the pre and postoperative assessment [75]. The attachment point, the extent of the tumor, the number of tumors, and relationship to surrounding structures, should all be evaluated carefully prior to surgical resection. Following surgery, it is important for TEE assessment to include evaluation of the left atrium and interatrial septum, in order to determine completeness of tumor resection as well as the integrity of the mitral valve. Atrial myxomas are best visualized by TEE with the mid esophageal views, using several multiplane angles to determine the location and extent of the tumor (Fig. 16.13, Video 16.13). Color flow and spectral Doppler should be used to evaluate any obstruction to mitral valve inflow, and/or mitral regurgitation resulting from damage to the mitral valve leaflets or valve interference by the tumor.
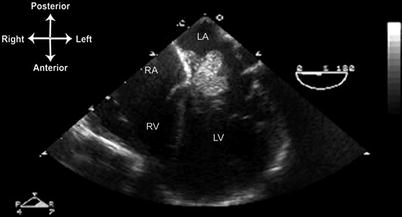

Fig. 16.13
Left atrial myxoma, seen from mid esophageal four chamber view, multiplane angle 0°. A large, lobulated myxoma is attached to the interatrial septum just posterior to the aortic root. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
Other Cardiac Tumors
Hemangiomas and teratomas are two other primary tumors that are occasionally encountered in the pediatric population. Hemangiomas can be located anywhere within the heart, but have a predilection for the right atrium and ventricular septum [76]. By echocardiography, there are numerous echolucent spaces, and color flow Doppler often demonstrates multiple vascular channels (Fig. 16.14, Video 16.14). Teratomas are usually detected in the fetus and neonate. They most commonly occur in the pericardial space, but can also arise within the heart, attached to the atrial or ventricular wall. When in the pericardial space, they are often attached to the aortic root or pulmonary trunk, and they can grow to a large size. There may be an associated pericardial effusion. If sufficiently large, either the tumor or the effusion (or both) can compress the heart, leading to tamponade physiology. When intracardiac in location, these can cause clinical signs and symptoms similar to other intracardiac tumors. By echocardiography, the tumors appear heterogeneous and encapsulated [67].
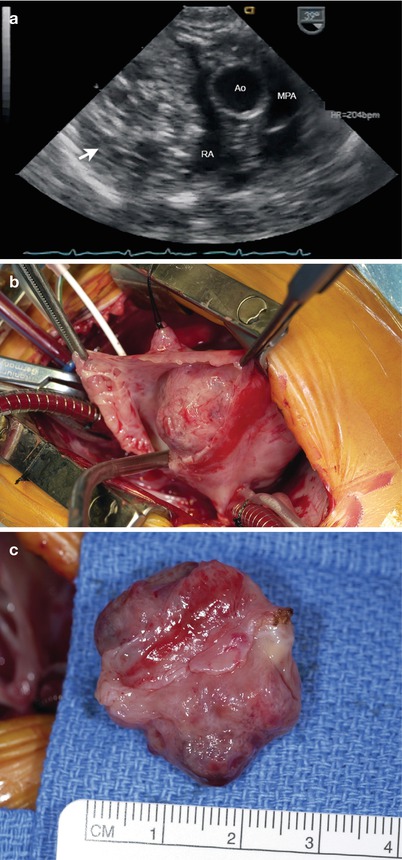

Fig. 16.14
Right atrial hemangioma as seen from a modified mid esophageal right ventricular inflow-outflow view. (a) Note the markedly heterogeneous nature of the large mass in the right atrium (arrow). The patient underwent surgery and the tumor was removed (b and c). Ao ascending aorta, MPA main pulmonary artery, RA right atrium
Role of TEE in the Evaluation of Cardiac Tumors
Most cardiac tumors are well seen by TTE, particularly in younger patients, and therefore TTE serves as the principal modality for their evaluation and follow-up. Cardiac magnetic resonance imaging has become increasingly utilized for diagnostic evaluation of cardiac tumors [77–79]. The role of TEE is primarily for two settings: (a) for those patients in whom TTE imaging is poor or incomplete, and (b) for intraoperative assessment. Specifics of optimal TEE probe location and multiplane angles, along with methods of evaluation, will depend upon the type and location of the tumor (as detailed above). In adults, TEE has been reported to provide more precise depiction of tumor attachment sites and extent of myocardial/pericardial involvement [80, 81]. Intraoperatively, the preoperative TEE study is used to confirm the nature and extent of tumor involvement [82, 83]. The postoperative TEE study evaluates the results of cardiac surgery—focusing on completeness of tumor resection residual obstruction (if previously present), whether any adjacent cardiac structures (e.g. cardiac valves) might have been affected by the resection, and assessment of ventricular function [84, 85].
Evaluation of Prosthetic Valves
Prosthetic valves are integral to the medical and surgical management of pediatric and adult patients with CHD. These valves fall into two major categories—mechanical and biologic—based upon valve composition (Table 16.6).
Mechanical heart valves contain nonbiologic materials (polymers, metal, carbon) in all parts of the prosthesis: the valve ring, sewing cuff, and orifice occluder. A number of mechanical valves have been developed over the past 50 years, essentially of three major types. The first type to be developed was the caged ball valve, consisting of a silastic ball with a circular sewing ring and a cage formed by three metal arches. The most notable of these was the Starr-Edwards valve (Fig. 16.15c), though similar valves have been produced including the Smeloff-Cutter valve. While these valves are no longer implanted, thousands of patients who received these valves are still alive, and continue to be followed regularly. The second type of mechanical valve is the monoleaflet valve, in which a single disk is secured by lateral or central metal struts, and surrounded by a sewing ring. The disk, generally made of extremely hard carbon (pyrolytic carbon), opens by tilting at an angle (about 60°–80°), resulting in two orifices of different sizes. Typical examples of this include the Bjork-Shiley (discontinued), and the Medtronic-Hall valve (Fig. 16.15b). The third major type of mechanical valve is the bileaflet tilting disk valve, made of two semicircular pyrolytic carbon disks attached by hinges to a rigid valve sewing ring. In the open position the valve leaflets tilt to an opening angle of 75°–90°, resulting in three orifices: a small slit-like orifice centrally between the two leaflets, and two semicircular orifices laterally. Of the three types of mechanical valves, the bileaflet tilting disk valve provides the most natural blood flow, greater effective orifice area for a given valve size, and it is also the least thrombogenic. Currently, they are the most commonly implanted mechanical valves, notably the St. Jude Medical (Fig. 16.15a) and Carbomedics bileaflet tilting disk valves. The latter two valves are available in a variety of sizes (from 16–33 mm) suitable for both pediatric and adult patients. The mechanical valves have a proven record of durability, however they require ongoing anticoagulation therapy, and there are ever-present risks of thrombosis and endocarditis of the valve. In many types of mechanical valves, separate aortic and mitral versions are available; nonetheless for a number of the mechanical valves, implantation has been performed in any of the four valve positions.
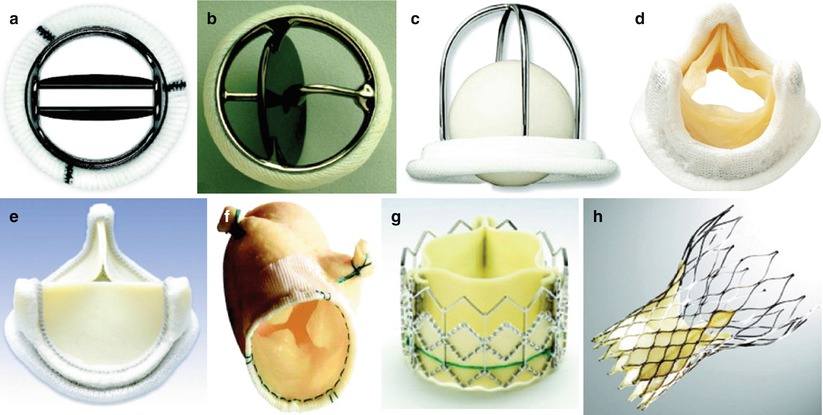
Table 16.6
Typical biologic and mechanical valves
Valve name/type | Manufacturer | Valve type/origin |
|---|---|---|
Biologic—Human | ||
Autograft | Pulmonary autograft | |
Allograft (Homograft) | Cryolife | Harvested cadaveric aortic, pulmonary homograft |
Monocusp, bicuspid | Surgically handsewn valve using autologous pericardium | |
Biologic—Heterograft | ||
Stented | ||
Hancock II | Medtronic | Porcine |
Mosaic | Medtronic | Porcine |
Carpentier-Edwards | Edwards-Lifesciences | Porcine |
Epic | St Jude | Porcine |
Biocor | St Jude | Porcine |
Trifecta | St Jude | Bovine pericardial |
Carpentier-Edwards Perimount Magna | Edwards Lifesciences | Bovine pericardial |
Mitroflow | Sorin Biomedica | Bovine pericardial |
Soprano | Sorin Biomedica | Bovine pericardial |
Stentless | ||
Freestyle | Medtronic | Porcine |
Toronto SPV | St Jude | Porcine |
Prima Plus | Edwards Lifesciences | Porcine |
Pericarbon Freedom | Sorin Biomedica | Bovine pericardial |
3 F Therapeutics Stentless Equine | 3 F Therapeutics | Equine pericardial |
Mechanical | ||
Starr-Edwards | Edwards Lifesciences | Ball-in-cage |
Bjork-Shiley | Pfizer | Single leaflet tilting disk |
Medtronic-Hall | Medtronic | Single leaflet tilting disk |
St. Jude Medical | St Jude | Bileaflet tilting disk |
CarboMedics | Sorin-CarboMedics | Bileaflet tilting disk |
ATS Medical | ATS Medical | Bileaflet tilting disk |
On-X | On-X Life Technologies | Bileaflet tilting disk |
Percutaneous—Biologic | ||
Melody | Medtronic | Bovine jugular valve mounted on platinum-iridium stent |
SAPIEN | Edwards Lifesciences | Bovine pericardium leaflets mounted on stainless steel or cobalt chromium alloy (SAPIEN XT) |
CoreValve | Medtronic | Porcine pericardium leaflets mounted on self-expanding nitinol frame |
Other | ||
SynerGraft | Cryolife | Tissue engineered decellularized allograft heart valve |
Contegra | Medtronic | Valved conduit of bovine jugular vein |

Fig. 16.15
Different types of prosthetic valves. (a) Bileaflet mechanical valve (St. Jude); (b) monoleaflet mechanical valve (Medtronic Hall); (c) caged ball valve (Starr-Edwards); (d) stented porcine bioprosthesis (Medtronic Mosaic); (e) stented pericardial bioprostheses (Carpentier-Edwards Magna); (f) stentless porcine bioprosthesis (Medtronic Freestyle); (g) percutaneous bioprosthesis expanded over a balloon (Edwards SAPIEN); (h) self-expandable percutaneous bioprosthesis (CoreValve). See text for details (From Pibarot and Dumesnil [95]; with permission of Walters-Kluwer)
Biologic heart valves are derived from human or animal tissue, and certain types contain nonbiologic material as well, such as metal and fabric. Human tissue valves fall into two categories: homografts (allografts) and autografts. Homograft valves are cryopreserved human cadaveric aortic and pulmonary valves, generally used as pulmonary or aortic valve replacements (Fig. 16.16). They come in a variety of sizes, depending upon donor availability. In contrast, an autograft represents the patient’s own valve translocated from one site to another. Usually, the autograft is the patient’s pulmonary valve translocated to the aortic position (Ross procedure) or rarely the mitral position (Ross II), with a homograft valve being placed in the original pulmonary position [86–88]. Biologic valves derived from animal tissue are known as xenograft (or heterograft) valves; the most commonly used animal tissues are porcine aortic valve and bovine pericardium, and the tissues are fixed with glutaraldehyde. These valves come in two major forms. Stented biologic valves contain a sewing ring and struts composed of nonbiologic material (metal, cloth), and valve tissue is sewn onto the fabric covering the struts. Both porcine valve (Fig. 16.15d) and bovine pericardium (Fig. 16.15e) are used with these types of valves. Stentless biologic valves contain no struts or sewing ring, which leaves more room for blood flow. Stentless xenograft valves derive primarily from harvested porcine aortic valves (Fig. 16.15f). Of note, human homograft and autograft valves also fall into the category of unstented biologic valves, since they contain no sewing ring or struts. This is because the entire homograft/allograft root (containing the valves) is harvested, thus the intrinsic structural support for the valve leaflets remains intact. Another category of bioprosthesis that has gained popularity is the Contegra pulmonary valve conduit. The Contegra conduit is a bovine jugular vein preserved in glutaraldehyde, and it contains a valve with three leaflets; the leaflets are similar to a human semilunar valve (Fig. 16.17). Since it is derived from a venous vascular structure, it is felt to be best suited for conditions of lower pressure such as the pulmonary circuit, and therefore it is used primarily for congenital heart surgeries in which a right ventricle to pulmonary artery conduit is needed such as the Ross procedure, tetralogy of Fallot, truncus arteriosus, etc. [89]. Thus it serves as an alternative to the homograft, and has achieved comparable short to intermediate term results [90–92]. Strictly speaking, it is a valved conduit (not solely a biologic valve), but it is used in a number of operations in which a valve is necessary. The Contegra conduit is available in both a supported and unsupported model; the supported model contains two external cloth covered polypropylene rings that encircle the valve annulus and commissure to provide additional support (Fig. 16.17a), and is utilized in those cases in which sternal compression could be an issue. As will be discussed below, the bovine jugular valve is also used for transcatheter valve technology.
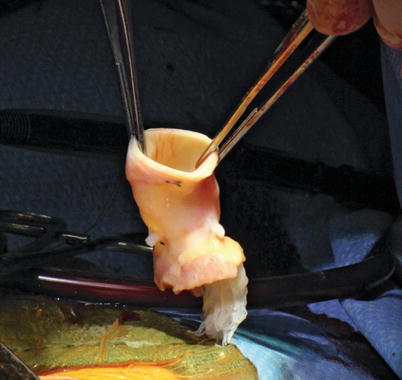
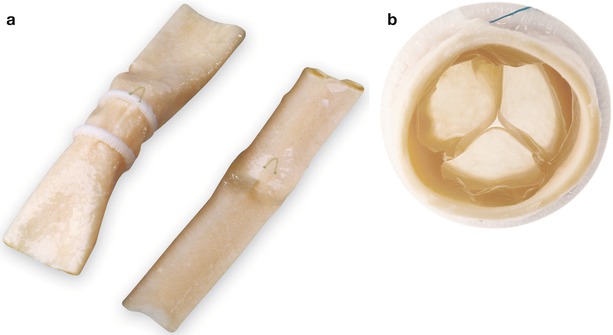

Fig. 16.16
Aortic homograft, following thawing and prior to implantation as a right ventricle to pulmonary artery conduit

Fig. 16.17
Contegra pulmonary valve conduit. The Contegra conduit is a bovine jugular vein with a trileaflet valve that is similar to a human semilunar valve. (a) The conduit is available in supported and unsupported models. The supported model contains two external cloth covered polypropylene rings that encircle the valve annulus and commissure to provide additional support for the valve housed inside the conduit. (b) Cross-sectional (en face) view of the trileaflet bovine valve inside the conduit (Image published with permission from Medtronic, Inc)
Over the years, a large number of different valve prostheses (both mechanical and biologic) have been developed, in a variety of valve sizes and profiles. Development continues at a rapid pace, with novel alternatives currently in development or advanced clinical trials. A list of some of the better-known biologic and mechanical valves is given in Table 16.6; this list is by no means exhaustive, and new models and types are periodically being introduced. A full discussion and elaboration of the many individual valves (including details about durability and complications) would require a separate chapter. For further information, the reader is referred to a number of references providing more detailed coverage of the topic [93–97].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


