Acute Myocardial Infarction
INTRODUCTION AND EPIDEMIOLOGY OF ST-ELEVATION MYOCARDIAL INFARCTION
This chapter focuses on the diagnosis and management of ST-elevation myocardial infarction (STEMI). STEMI represents the most urgent presentation among the acute coronary syndromes (ACSs). This condition mobilizes a health care network with the aim of promptly restoring coronary perfusion in order to improve myocardial salvage and patient survival. This is a distinct clinical entity from unstable angina (UA) and non–ST-elevation myocardial infarction (NSTEMI), which were discussed in the previous chapter. In contrast to UA, which is characterized by ST depressions or T-wave inversions without elevated cardiac biomarkers, and NSTEMI, which is characterized by elevated cardiac biomarkers without ST-segment elevations, STEMI usually presents with ST elevations that are localizing in the territory of the infarcted myocardium.
Acute myocardial infarction (AMI) or onset of angina is the usual initial clinical presentation for coronary disease, although about 20% of individuals with a coronary event do not even present to the hospital. For these individuals, sudden cardiac death (SCD) due to ischemia-triggered ventricular fibrillation (VF) is the initial manifestation of symptomatic coronary disease. Unless defibrillation occurs within minutes, death ensues quickly. Fortunately, automated external defibrillators have become more available and are now found in many public places. Rates of neurologically intact survival for out-of-hospital cardiac arrest due to VF, are highly variable across the United States and are dependent on early defibrillation, effective bystander cardiopulmonary resuscitation (CPR), prompt emergency medical services (EMS) intervention, rates of revascularization, implementation of hypothermia protocols, and adequate postarrest care.
More than half a million hospital presentations per year are attributable to STEMI. This condition is responsible for higher in-hospital mortality than UA or NSTEMI. Whereas the early management decision in UA/NSTEMI is deciding whether a patient should be directed to an early invasive approach versus conservative management, in STEMI, the focus is on rapid pharmacologic or mechanical reperfusion.
Issues such as risk stratification, fibrinolysis, primary percutaneous coronary intervention (PCI), adjunctive medical therapy, and discharge planning are discussed in the following text. A controversial issue is whether patients with suspected AMI should be directed to the nearest hospital or to a facility with cardiac catheterization and surgical capabilities. Important patient characteristics and logistical considerations are reviewed that may favor one approach over another. This chapter follows the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.
CLINICAL PRESENTATION
STEMI typically presents with substernal chest discomfort that is described as a pressure or heavy sensation that lasts more than 30 minutes. Symptoms are often described as “vicelike” or “an elephant sitting on my chest.” Patients may display the Levine sign by clutching their fist over their chest. STEMI is often accompanied by dyspnea, nausea, vomiting, and diaphoresis. Atypical symptoms are more common in diabetics, women, and the elderly (similar to UA/NSTEMI patients). There is a subset (~20%) of patients who have myocardial infarction (MI) in the absence of clinically recognized symptoms. Successful reperfusion is dependent on early recognition of symptoms by the patient with prompt activation of the EMS. As a goal, EMS personnel should arrive at the subject’s location within 10 minutes of system activation. Unfortunately almost 40% of patients with STEMI fail to activate EMS. EMS-transported patients have significantly shorter delays in both symptom onset to arrival as well as door to reperfusion time.
DIAGNOSIS
The sine qua non for the diagnosis of STEMI is recognizing ST elevations in a typical coronary distribution or a new left bundle branch block (LBBB) in the setting of typical (or atypical) symptoms. In the NCDR ACTION registry, performance and transmission of an out-of-hospital 12-lead EKG was associated with a greater and more timely use of reperfusion therapy with a trend toward lower mortality likely because it facilitates early activation of the STEMI protocol. Waiting for cardiac biomarkers to return before making a diagnosis of AMI and initiating emergency therapy is inappropriate (class III recommendation). A brief phase before ST elevations appear is often unrecognized. This phase is characterized by hyperacute T waves in the infarct-related territory. The hyperacute electrocardiogram (ECG) findings rapidly progress to typical ST elevations. ST elevations are usually convex or “tombstone” in appearance, although they can be concave. STEMI is diagnosed when at least 1-mm ST elevations are recognized in two or more contiguous leads. Figures 41.1 to 41.4 show various ECG examples of STEMI. Patients with STEMI in the posterior circulation can manifest with ST depression across the anterior precordial leads. In patients with initial nondiagnostic ECG findings, serial repeat EKGs are warranted.
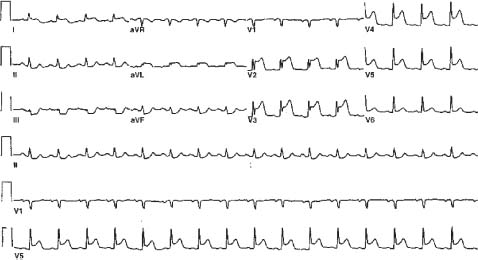
FIGURE 41.1 Anterior ST-elevartion AMI. There is also ST elevation in leads I and aVL, suggesting a left anterior descending artery occlusion proximal to a major diagonal branch.
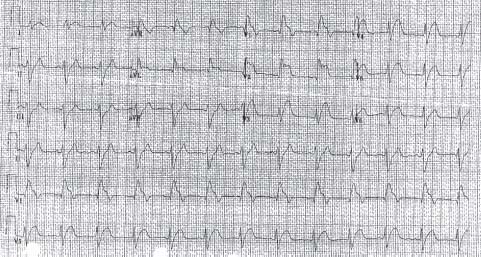
FIGURE 41.2 Anterior ST-elevation AMI. In addition to ST elevation in leads I and aVL, there is also QRS prolongation, suggesting a left anterior descending artery occlusion proximal to a major diagonal branch and a major septal perforator.
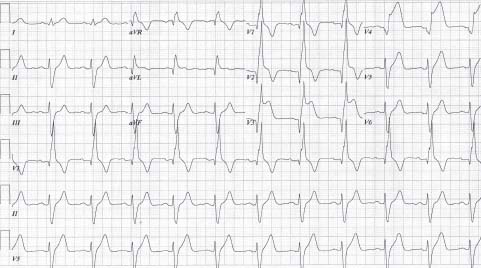
FIGURE 41.3 Anterior ST-elevation AMI in the setting of a preexisting RBBB. ST elevation is noted in leads V3 to V6depression and the concordance or discordance of the ST segment with the QRS.
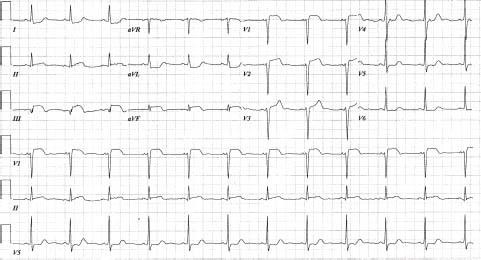
FIGURE 41.4 Inferior ST-elevation AMI. There is also ST elevation in leads V1 to V3, suggesting RV involvement. RV leads should also be done to confirm RV involvement (i.e., occlusion proximal to acute marginal branch).
The ECG can also help to localize the location of the coronary occlusion. For example, high lateral (i.e., I and aVL) ST elevations that accompany an anterior MI indicate a left anterior descending artery occlusion proximal to a major diagonal branch. An anterior STEMI with ST-segment elevation in lead V1 and QRS complex prolongation indicates a left anterior descending artery occlusion proximal to a major septal perforator. Most STEMIs (70% to 80%) eventually progress into Q waves in the region of the infarcted myocardium.
Although the ECG is diagnostic in the setting of STEMI, other conditions that cause ST elevations must be simultaneously screened for and evaluated. These include acute pericarditis, hyperkalemia, left ventricular hypertrophy, early repolarization, and ventricular aneurysm (Table 41.1).
TABLE
41.1 Differential for ST-Segment Elevation
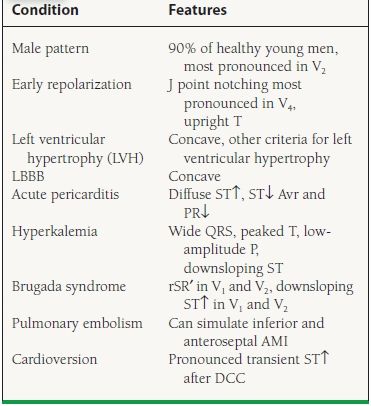
From Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349:2128–2135, with permission from the Massachusetts Medical Society.
A posterior MI is an important STEMI equivalent. This is often seen in the setting of an inferior or inferolateral MI. The ECG findings are a tall R wave in V1 with ST depressions in V1 to V2. It is critical to recognize an isolated posterior infarction as a STEMI, because patient prognosis hinges on the prompt restoration of coronary flow.
The other STEMI equivalent to consider is a new or presumably new complete LBBB. Not surprisingly, patients who present with a complete LBBB have high in-hospital mortality rates (up to 25%), in part due to the fact that they are nearly 80% less likely to receive reperfusion therapy than patients who present with recognizable ST elevations. However, even with reperfusion therapy, mortality rates are higher in patients with new complete LBBB than with ST elevation, attesting to the high-risk nature of this population. Although ischemic changes are interpretable in the context of a right bundle branch block (RBBB), this task becomes more difficult with a complete LBBB. There are criteria that can help diagnose a LBBB as an AMI with good specificity (Table 41.2), which look at the degree of ST elevation or depression and the concordance or discordance of the ST segment with the QRS.
TABLE
41.2 ECG Criteria for the Presence of AMI in the Setting of LBBB

Adapted from Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996;334:481–487.
RISK STRATIFICATION
Killip Class, TIMI Risk Score, GRACE Score, ACTION-GWTG Risk Score
Since all patients with STEMI are initially eligible for reperfusion therapy, risk models are used primarily to determine prognosis and not to direct therapy as in UA/NSTEMI risk models. Initial information for risk stratification comes from the physical exam. Assessing for signs of heart failure is a useful tool for risk stratification. Patients who present with cardiogenic shock have a 30-day mortality rate of approximately 60% (Table 41.3). Cardiac biomarkers (troponin I or T and total CK and CK-MB isoenzyme) supplement the physical exam by gauging infarct size and providing additional prognostic information.
TABLE
41.3 Killip Class—30-Day Mortality
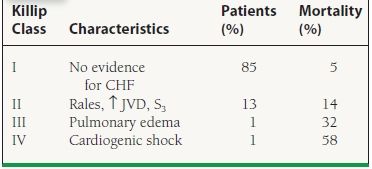
From Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91:1659–1668, with permission from Wolters Kluwer Health.
Risk models have been created that provide clinicians with a more accurate prediction of risk. These models combine multiple variables that are most predictive for future adverse cardiac outcomes. The thrombolysis in myocardial infarction (TIMI) risk score is an easily used and validated model that has important prognostic implications. It incorporates eight variables that are readily available from the history, physical exam, and ECG (Fig. 41.5). In a fibrinolytic treated population, a TIMI risk score of greater than eight predicts an approximately 35% incidence of death at 30 days. This is in contrast to a score of zero to one, for which the 30-day mortality rate is <2%. The strongest variable that predicts an adverse prognosis is advanced age (where age ≥75 years receives 3 points and age 65 to 74 years receives 2 points). Other variables include hypotension, tachycardia, or Killip class II to IV at presentation, history of diabetes or hypertension, low body weight, anterior ST elevation (also complete LBBB), and a time to treatment of >4 hours.
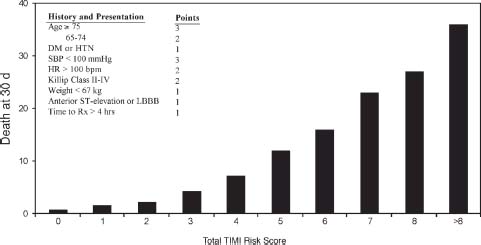
FIGURE 41.5 TIMI risk model for prediction of short-term mortality in STEMI patients. (From Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–2037, with permission from Wolters Kluwer Health.)
The GRACE score was designed to improve the risk prediction of in-hospital mortality in patients with ACS. It can be used in patients with ST-elevation and non–ST-elevation myocardial infarction. Risk is determined based on Killip class, systolic blood pressure, heart rate, age, creatinine level, presence or absence of cardiac arrest at admission, ST-segment deviation, and presence or absence of cardiac biomarkers. A score of ≤60 is associated with a ≤0.2% probability of in-hospital mortality whereas a score of ≥250 is associated with a ≥52% probability of in-hospital mortality.
A mortality model and risk score utilizing a contemporary set of patients is that derived from the ACTION Registry. In this validated model, patients with a risk score of <40 had an observed mortality rate of 4% compared with a 12% observed mortality rate in subjects with a score >50.
MANAGEMENT
Initial Approach
The initial assessment of a patient suspected of having an AMI is to establish intravenous access and start supplemental oxygen for individuals who are hypoxic or who show signs or respiratory distress. Simultaneously, a targeted history and physical exam should be obtained. The history and physical exam provide prognostic information, but also can suggest an alternative diagnosis and help identify mechanical complications of STEMI. It is important to rule out other causes of chest pain such as aortic dissection. Pain arising from a gall stone, renal stone, pancreatitis, esophageal dys-motility, pneumothorax, pleuritis as well as impending herpes zoster may frequently mimic this presentation.
If reperfusion with fibrinolysis is considered, the history and physical exam should screen for contraindications to its use. Because the most feared complication with the use of fibrinolytics is intracranial hemorrhage (ICH), patients with an increased risk for this complication must be identified. Risk factors for ICH are advanced age, female gender, uncontrolled hypertension, and low body weight. Patients with coagulopathies (e.g., patients on Coumadin therapy) are also at increased risk for bleeding. Absolute and relative contraindications to fibrinolysis are listed in Table 41.4.
TABLE
41.4 Contraindications to Fibrinolysis
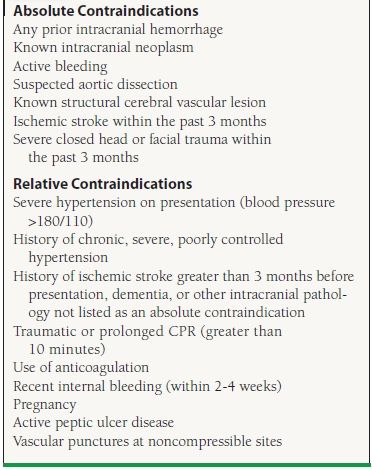
Adapted from Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction-executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:671-719.
Reperfusion Therapy
Time is of paramount importance in reinstituting coronary flow. The greatest improvement on mortality comes from reperfusion within the first hour, the so-called golden hour. Reperfusion therapy can be considered up to 12 hours from the onset of chest pain and even longer in select cases. In order to facilitate rapid coronary reperfusion, a pharmacologic or mechanical approach should be decided on quickly. The current goal for door-to-lytic time is 30 minutes, whereas the goal for door-to-balloon time is 90 minutes if the patient presents to a PCI capable facility and 120 minutes if the patient requires transfer for PCI.
In general, if primary PCI can be performed immediately (i.e., the patient presents to a center capable of performing PCI), this is the preferred choice for reperfusion (class I recommendation). This information comes from a metaanalysis of 23 trials that randomized nearly 8,000 STEMI patients to fibrinolytic therapy versus primary PCI. The hospitals included in the analysis were largely experienced providers of coronary intervention and were able to deliver mechanical reperfusion in a timely fashion, although some studies enrolled patients who were transferred for primary PCI versus given immediate fibrinolysis. This study was a contemporary analysis, as stents were used in 12 of the trials and glycoprotein (GP) IIb/IIIa inhibitors were used in eight. General inclusion criteria required that patients have ischemic symptoms within the previous 6 to 12 hours and at least 1-mm ST elevations in contiguous leads or a new/presumable new complete LBBB. Patients also needed to be candidates for fibrinolysis to be eligible for enrollment. A notable exception was the SHOCK trial, as this study enrolled patients with cardiogenic shock and chest pain within the preceding 36 hours. Since the SHOCK trial was the outlier to the overall analysis, the analysis was performed with and without this study. Most patients (76%) received fibrin-specific (i.e., t-PA) lytic agents, whereas the remainder received streptokinase. This analysis revealed a short-term survival advantage as well as a reduction in recurrent MI and hemorrhagic stroke in those who received primary PCI. Short-term mortality was 7% in the primary PCI group, compared to 9% in the fibrinolytic group (p = 0.0002). Long-term mortality was also significantly reduced (p = 0.0019). Thus, among patients who present within 12 hours of the onset of chest pain to a tertiary care center that is capable of performing primary PCI expeditiously, data supports the use of mechanical reperfusion.
When individuals present to a community hospital without primary PCI capabilities, the question becomes whether to transfer the patient to a primary PCI center or to administer immediate fibrinolysis. Fibrinolysis is limited by postlysis TIMI 3 flow of <50% at 90 minutes and risk of reocclusion, which results in inadequate myocardial salvage and heightened rates of recurrent ischemia and reinfarction. There is also a definite risk for ICH (up to 0.9% in many trials and even greater in high-risk patients). There are also numerous contraindications to consider. These limitations have led to trials that specifically addressed if delaying immediate reperfusion to allow transfer for primary PCI may be beneficial.
A subanalysis from the previously mentioned metaanalysis examined the studies that transferred patients for primary PCI versus giving immediate fibrinolytics. The mean time that was required for transfer to a primary PCI center was 39 minutes. Mortality was similar between the two groups (p = 0.057), although a composite outcome that included death, reinfarction, or stroke was reduced by transfer for primary PCI (p < 0.001).
Another meta-analysis specifically addressed this issue using available clinical trial information. This study examined only trials that randomized patients to either immediate fibrinolysis or transfer to a center capable of performing primary PCI. The inclusion criteria were similar to the previous meta-analysis: acute STEMI within 6 to 12 hours from the onset of chest pain and eligibility to receive a fibrinolytic agent. Six trials were available for analysis, involving nearly 4,000 patients. A few of these trials deserve special comment. The AIR-PAMI study randomized patients who were high risk to one of the above reperfusion strategies. High risk was defined as age >70 years, heart rate >100 beats/min (bpm), systolic blood pressure <100 mm Hg, Killip class II/III, complete LBBB, or anterior MI. Although this was the smallest study included in the meta-analysis, there was no noticeable harm in transferring high risk patients for PCI. The CAPTIM trial was unique in that patients were randomized to a reperfusion strategy before arrival to the hospital, which enabled fibrinolytics to be given in an even more timely fashion. This was the only trial that showed a nonsignificant trend in mortality favoring fibrinolysis. The PRAGUE-2 trial examined the optimal reperfusion strategy based on time from the onset of chest pain. The study was stopped prematurely, as mortality was increased 2.5-fold among patients who presented more than 3 hours from the onset of chest pain who received fibrinolysis (15% with fibrinolysis and 6% with primary PCI, p < 0.02). In Patients who presented within 3 hours from the onset of chest pain, mortality was similar between the two reperfusion strategies (7.4% with fibrinolysis and 7.3% with primary PCI).
So, while transferring a STEMI patient for primary PCI versus immediately administering fibrinolysis is controversial, some patient characteristics and logistical considerations favor one approach over another. According to the 2011 ACCF/AHA/SCAI guidelines for PCI, if a patient presents with STEMI and can undergo PCI within 120 minutes of first medical contact, this is the preferred approach. Conversely, if the patient cannot receive PCI within 120 minutes of first medical contact, and there are no contraindications to fibrinolysis, fibrinolytics should be administered within 30 minutes of hospital presentation (class I recommendation). It is important to note that the effectiveness of fibrinolytics are highly time dependent with a marked efficacy when administered in the first hour following STEMI onset. Accordingly, for patients who are at high risk for bleeding or who present more than 3 hours after the onset of chest pain, transfer for primary PCI is favored. Additionally, patients who are in cardiogenic shock benefit from mechanical revascularization, but have not been shown to have a mortality reduction with fibrinolysis. Lastly, in the elderly who have an increased risk of ICH, patients with contraindications to fibrinolytics or when the diagnosis of STEMI is in doubt, PCI should be considered. When interhospital transfer for primary PCI is planned, the DIDO (door in to door out) time at the originating hospital is an important performance measure and should ideally be <30 minutes. This benchmark is associated with optimal door to balloon times and lower in-hospital mortality.
Fibrinolytic Therapy
A large body of research involving tens of thousands of patients documented the benefit of fibrinolytic therapy in reducing infarct size, preserving left ventricular function, and improving survival in AMI patients. For every 1,000 patients treated with fibrinolytics within 1 hour of onset of symptoms, the number of lives saved is 26, while treatment within 3 to 6 hours from the onset of chest pain saves 18 lives. There is still a survival advantage from 6 to 12 hours, although it is smaller in magnitude than giving lytics closer to the onset of chest pain. Accordingly, fibrinolysis is indicated for 1 mm or more of ST elevations in contiguous leads, or a new complete LBBB within 12 hours from the onset of chest pain. Patients with stuttering infarcts may benefit from lytics up to 24 hours after the onset of chest pain. Asymptomatic patients more than 24 hours out from the onset of chest pain should not receive lytic therapy (class III recommendation).
If lytic therapy is selected, it is important to know the different agents used for fibrinolysis and which are available at a given institution. Additionally, contraindications to the use of fibrinolytics should be reviewed in every eligible patient. The choice of one agent over another is made according to hospital availability and physician experience with a given agent.
Streptokinase, a first-generation fibrinolytic agent, is capable of lysing circulating and clot-bound fibrin. Allergic reactions are common, and reexposure to streptokinase should be avoided. This is the least expensive lytic agent, at around $500 per dose. Streptokinase may not require adjunctive heparin therapy unless the patient is at high risk for emboli (i.e., atrial fibrillation or known left ventricular thrombus). Accordingly, lysis with streptokinase is associated with a slightly less ICH risk (0.5% compared to 0.7% for fibrin-specific agents). This property makes streptokinase attractive if an individual is not a candidate for PCI and is at high risk for ICH. An example would be a small, elderly, hypertensive female with a history of a remote ischemic stroke who presents with an extensive anterior MI and refuses PCI.
Fibrin-specific agents activate plasminogen directly and are relatively selective against clot-bound fibrin rather than circulating fibrinogen. Allergic reactions do not occur with these agents, as can occur with streptokinase. Fibrin-specific agents include alteplase (tPA), reteplase (rPA), and tenect-eplase (TNK-tPA). Because these fibrin-specific agents do not produce a systemically lytic state and because they activate platelets, the use of heparin therapy appears to improve and maintain vessel patency.
The GUSTO-I trial was a landmark study published in 1993 that compared streptokinase to various fibrin-specific strategies. Up until this trial there was no known advantage of one agent over another. This trial studied >40,000 patients with AMI and revealed the superiority of accelerated tPA over streptokinase. Accelerated tPA with intravenous heparin resulted in a 14% reduction in mortality and higher rates of TIMI 3 flow at 90 minutes (54% vs. 31%) compared to streptokinase-based regimens. The accelerated tPA dose is a 15-mg bolus, then 0.75 mg/kg (up to 50 mg) over 30 minutes, followed by 0.5 mg/kg over 60 minutes (up to 35 mg).
Reteplase is less fibrin-specific than alteplase. This agent is equivalent to alteplase in terms of efficacy, although it is easier to administer (two 10-mg boluses administered 30 minutes apart). Tenecteplase is the easiest lytic to administer, because it is given as a single bolus (dose ranges from 30 to 50 mg, adjusted for body weight). See Table 41.5 for dosing. This agent is more fibrin specific and has a slower plasma clearance than the other fibrin-specific agents. The ASSENT 2 trials showed the noninferiority of tenecteplase compared to alteplase. In this trial, there was also less major bleeding with tenecteplase, and a trend toward less ICH in elderly women. Equivalent efficacy, enhanced safety, and ease of administration make tenecteplase an attractive fibrinolytic agent.
TABLE
41.5 Weight-based Dosing of Tenecteplase

Reprinted from Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction-executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:671–719, with permission from Elsevier.
Percutaneous Coronary Intervention
When PCI is selected for reperfusion, eligibility criteria are the same as those used for fibrinolytics: 1 mm or more of ST elevations in contiguous leads or a new/presumably new complete LBBB within 12 hours of the onset of chest pain. A posterior MI should be treated as a STEMI equivalent. The goal of PCI is to achieve optimal revascularization of the infarct-related artery by establishing TIMI 3 flow. Multivessel revascularization at the time of primary PCI is usually not indicated (class III recommendation), except in patients with cardiogenic shock.
Several approaches to PCI exist in the setting of STEMI. Most data support the use of primary PCI. In primary PCI, fibrinolytics are not given prior to intervention. Patients either present directly to a PCI center, or they are transferred (without fibrinolysis) from a community hospital to a center capable of performing PCI. As mentioned previously, the downside in transferring a patient for primary PCI is the delay in time that is required until mechanical reperfusion can occur.
In a pharmacoinvasive strategy patients who receive fibrinolysis are transferred for early PCI regardless of reperfusion status. The TRANSFER AMI trial evaluated this strategy in high-risk STEMI patients. In TRANSFER AMI, patients who received fibrinolysis followed by PCI within 6 hours of presentation had a 6% absolute and 46% relative reduction in the composite endpoint of death, reinfarction, recurrent ischemia, heart failure, and shock when compared with patients who received fibrinolysis followed by rescue or delayed PCI. Based on these results, patients who are high risk and receive fibrinolysis as the primary reperfusion strategy should be transferred to a PCI-capable facility as soon as possible (class IIa recommendation). Table 41.6 lists the criteria for defining high-risk patients. For low-risk patients this same management strategy is considered a class IIb recommendation.
TABLE
41.6 ACC/AHA Recommendations for Triage and Transfer for PCI: High-risk Definition
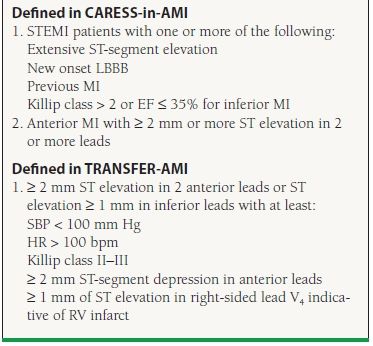
Adapted from Kushner FG, Hand M, King SB, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (Updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (Updating the 2005 Guideline and 2007 Focused Update). J Am Coll Cardiol. 2009;54:2205–2241.



