Characteristic
Incidence (percent)
Median age
75 years
Female
More than 50 %
Coronary artery disease
60 %
Hypertension
70 %
Diabetes
40 %
Atrial fibrillation
30 %
Renal insufficiency
30 %
Cardiogenic shock associated with an acute coronary syndrome (ACS) is associated with a much worse prognosis. In an analysis of the Global Registry of Acute Coronary Events (GRACE) the presence of cardiogenic shock in patient with ACS was associated with a 59 % in-hospital mortality rate. For those patients with ACS who did not develop cardiogenic shock, the in-hospital mortality rate was only 2.3 % [4]. What the GRACE registry also tells us is that cardiogenic shock associated with ACS is relatively occurs at a relatively low rate, 4.6 %. Of those patients with ACS, 57 % underwent cardiac catheterization and 47 % had revascularization. The presence of cardiogenic shock did cause the patients treatment with recommended medication to be changed [4]. So while developing cardiogenic shock after ACS is uncommon, the risk of death when it does occur is significant.
Medical Management and Referral
Often times, acute cardiogenic shock treatment begins with the use of single the multiple inotropes and vasopressors. This escalation often then leads to the placement of an intra-aortic balloon pump (IABP). While a recent article of the results of the IABP-SHOCK II trial call into question the benefit of IABP use in patients with cardiogenic shock from ACS who are planned for early revascularization [5], its use is currently in the algorithm.
For those patients with refractory cardiogenic shock, the associated early mortality rate is more than 50 %. This is even in the setting of being adequately revascularized [6, 7]. In those patients who are refractory to medical management, acute MCS is often the only means of survival. However, these patients are exceedingly ill and prone to a high degree of liability. This compromise extends to the hemodynamic blood pressure regulation, coagulation, hepatic and renal perfusion, and any subsequent multi-system organ failure [8].
There is a role for aggressive, early use of MCS. In a series of 41 patients with refractory shock treated at the University of Minnesota with placement of central, centrifugal flow pump MCS, 68 % of patients were able to be discharged from hospital with only 19.5 % dying while being supported on MCS [8]. This approach allows for a bridge to recovery or decision and will be the basis for the remainder of our discussion.
Technology and Approaches
As previously mentioned, MCS is often the only option for survival in those patient with cardiogenic shock from ACS or in those with acute decompensated heart failure. This treatment algorithm is a short-term, temporary cardiopulmonary support that enables time for clinical decisions on long-term management to be made (Fig. 6.1). The use of MCS as a bridge to recovery or decision is, just that. The use of these technologies allow for improvement of end organ perfusion facilitate any recovery that would then enable explant of the MCS. The use of permanent left ventricular assist device (VAD) placement in the setting of multi-system organ failure and hemodynamic instability has a prohibitively poor outcome [9].
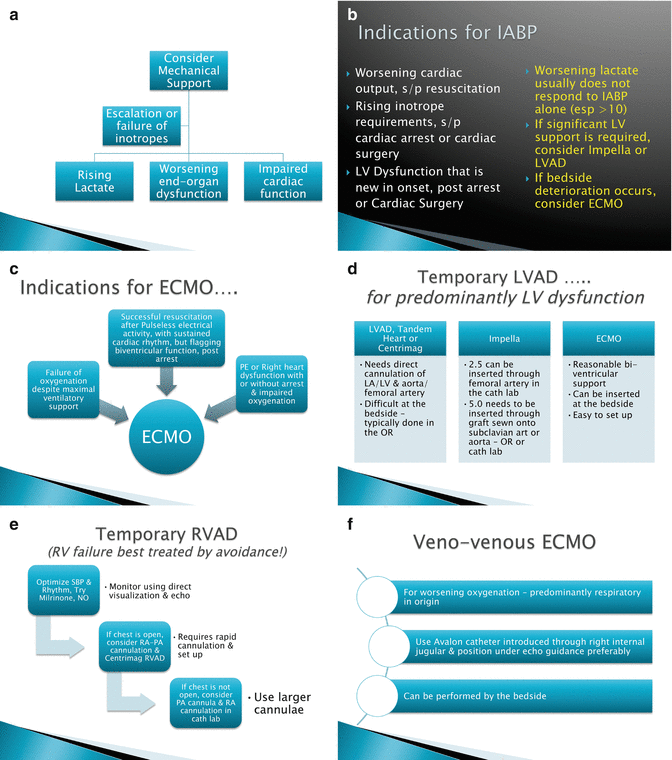

Fig. 6.1
(a–f) Algorithm for acute MCS
Therefore, the use of MCS to improve end-organ perfusion and more thoroughly resuscitate the patient allows the physicians, treatment team, support services, the patient and their family to discuss long-term options. If the patient does not recover end organ or neurologic function then plans for end of life care can be made. There are many options for short-term or acute MCS. There are surgically and percutaneously placed technologies. For clarity of nomenclature, either isolated uni- or bi-ventricular support is termed a VAD. The placement of an in-line membrane oxygenator, in either central or peripheral VAD, then is termed extra-corporeal membrane oxygenation (ECMO) or extra-corporeal life support (ECLS).
Surgically Placed Ventricular Assist Devices
Thoratec CentriMag
The Thoratec CentriMag (Thoratec, Pleasanton, CA, USA) pump system (Fig. 6.2a) is an extracorporeal centrifugal blood pump. The system, initially introduced and provided by Levitronix (Wiltham, MA, USA) and subsequently acquired by Thoratec, contains a centrifugal blood pump, a motor, a controller console, and a flow probe [8, 10, 11]. A key advantage of the CentriMag pump, as compared to other centrifugal pumps on the market, is that has a low risk of hemolysis and thrombosis formation. This risk is ameliorated through the magnetically levitated impellar and a lack of bearings or seals. These features also produce minimal wear. A flow rate of up to 10 L/min can be obtained over pump speeds that range from 500 to 5,500 rpm [8, 9, 12].
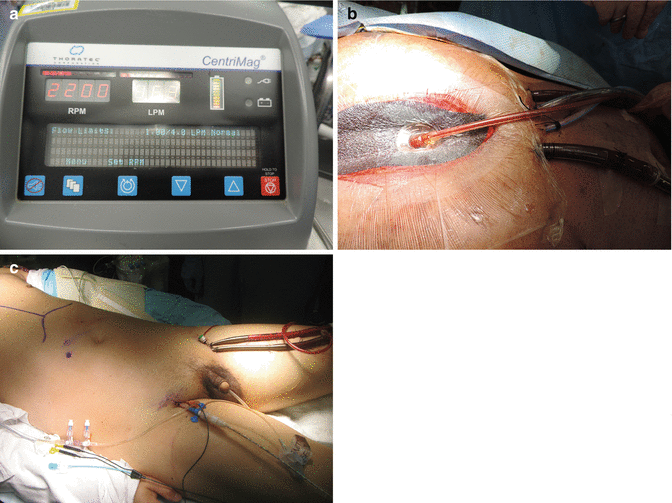

Fig. 6.2
(a) Schematic picture of CentriMag. (b) Central Cannulation with CentriMag. (c) Peripheral cannulation with CentriMag
There is a great deal of versatility in the implantation technique of the CentriMag which allows the surgeon to tailor the approach to best fit each patient’s clinical situation. It may be implanted centrally or peripherally and has the ability to provide single or biventricular support. The ventricular support can be either right (RVAD) or left (LVAD). Biventricular support (BIVAD) can be obtained by utilizing two pumps, one for each ventricle. An oxygenator can easily be spliced in-line to convert to ECMO at any time, either intraoperatively or postoperatively.
For central cannulation (Fig. 6.2b), a median sternotomy is performed. Cardiopulmonary bypass (CPB) may or may not be utilized. In the patient with a large body habitus or intolerance of holding respiration, CPB may add a degree of safety to the cannulation operation. Peripheral cannulation with cannulae inserted percutaneously through the femoral artery and vein (Fig. 6.2c) provide a very satisfactory method of supporting these sick patients emergently. This can be performed by the bedside.
For LVAD implantation (Fig. 6.3), the inflow cannula is placed into the left atrium through the right superior pulmonary vein at the interatrial septal groove. The outflow cannula is placed into the ascending aorta via direct cannulation as one would do for CPB. When used as an RVAD, the inflow cannula is placed in the right atrium, typically through the right atrial appendage. The outflow cannula is placed into the main pulmonary artery in a fashion similar to standard aortic cannulation. All cannula are secured with pledgeted purse string stitches (Fig. 6.4) and tourniquet. The purse strings are secured to the cannula. Of note, the cannula are ideally tunneled, as one would a chest tube, prior to insertion or connecting to the CentriMag circuit. Placement is characterized via trans-esophageal echocardiography [8].
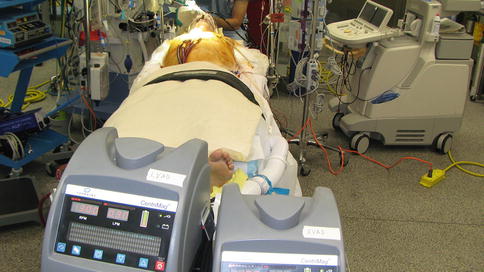
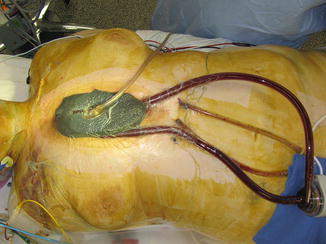

Fig. 6.3
Centrimag central – LVAD, RVAD, and BIVAD

Fig. 6.4
RVAD with diagram of pledgets for cannulation
For peripheral cannulation, only a LVAD approach can be utilized. The peripheral cannulation is typically via the femoral vessels (Fig. 6.5). The femoral artery and vein can be accessed directly. If the femoral artery has the arterial cannula placed into it, the distal limb perfusion should be assessed and if necessary distal limb perfusion implemented. Alternatively, the femoral artery may be accessed via placement of a 8-mm “chimney” graft onto the femoral artery and the arterial inflow cannula placed into it. The use of a chimney graft, while a slightly longer operation, provides antegrade distal limb perfusion.
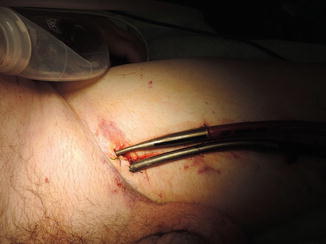

Fig. 6.5
Centrimag peripheral cannulation picture; inset showing peripheral cannulation via direct cannulation and chimney graft
The CentriMag pump allows for the support to be weaned over time or intermittently to assess for function as an evaluation of recovery, the need for long-term MCS, or transplantation. Reports have described use of a CentriMag system for over 100 days without pump failure or thromboembolic complications [13, 14]. The modular nature of the CentriMag, and/or membrane oxygenator, in this setting allow for relatively easy exchange at bedside, whether in the intensive care unit or the operating theater [8, 14].
After ensuring hemostasis, heparin is used to anti-coagulate the patient with a goal activated clotting time (ACT) of 180–220 s. If mediastinal bleeding occurs, the heparin can be held for up to 48 h if the flow is maintained at 4 L/min or more and there are no concerns for pump malfunction, thrombosis, or emboli [8, 9].
In the group at the University of Minnesota’s published series, the mean duration of CentriMag support in acute, refractory cardiogenic shock, was 12.2 days. The mortality rate with this device, in that series was 19.5 %. Of the 41 patients in the series, 68.3 % were able to be discharged from hospital. Sixteen of the patients were bridged to long-term MCS [8].
Abiomed BVS5000
The Abiomed BVS5000 (Abiomed, Inc., Danvers, MA, USA) is an external, pulsatile VAD. The BVS5000 is pneumatically controlled [15–17]. An external pneumatic drive console controls the system’s single use blood pump (Fig. 6.6). In the Abiomed system, there are dual chambers that reflect those in a heart; an atrial chamber to “fill” and a ventricular chamber to “pump”. The atrial chamber is passively filled throughout the cardiac cycle. The ventricular chamber then pumps extracorporeal blood back to the patient through ventricular chamber systole. The atrial chamber’s continuous drainage allows for continuous drainage of the heart. There are two versions of the pneumatic controller that enables up to 5 L/min, or 6 L/min of flow in the high flow version. The pneumatic pump empties when the chamber is sensed to be full so there is asynchrony between the pump and the patient’s cardiac cycle. This feature is potentially advantageous in patients with unfavorable ventricular rhythms [8].
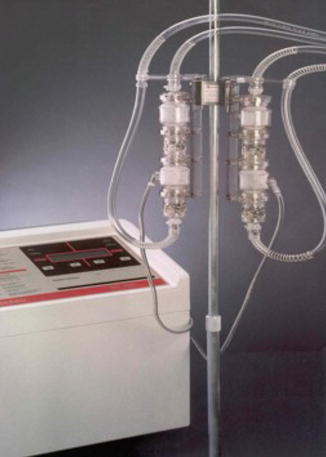

Fig. 6.6
Abiomed BVS 5000 – pump
The typical insertion of the Abiomed BVS5000 is via median sternotomy. As with the CentriMag, there is option of implantation on or off CPB and for the ability for uni-ventricular or BIVAD support [8]. There have been descriptions of alternative implantation strategies (posterolateral thoracotomy) [18]. In this system, the inflow cannula for LVAD and RVAD are placed similarly with cannula placed into the atrium. The outflow cannula utilize a graft sewn to the artery. For the LVAD application, the inflow cannula is placed into the left atrium via the superior pulmonary vein at the intraatrial groove, the left dome of the atrium, or the left atrial appendage. The cannula is secured again with double purse string sutures [17]. For the RVAD application, the inflow cannula is placed into the right atrium, typically via right atrial appendage. For the outflow in both LVAD and RVAD applications, a woven graft (10–14 mm) is anastomosed to the aorta (LVAD) or main pulmonary artery (RVAD) in an end-to-side fashion with running non-absorbable mono-filament suture [8, 16, 17, 19]. If need be, an oxygenator can be attached to the system for pulmonary support. As an alternative cannulation strategy, femoral access has been described [15, 20].
Anticoagulation should be initiated as soon as it is safe and the patient is free from ongoing bleeding (within 24 h). The anastomosis of the graft conduit to the artery is a potential source of bleeding and should be considered if there is a change in hemodynamic, chest tube output, or concern for trauma from traction on the pneumatic pump. The pump head needs to be monitored for fibrin build up or clot. If there is thrombus, the pump should be exchanged. The pump may go up to a week without needing to be exchanged [8]. In a series of BVS5000 patients, almost 50 % were able to be weaned from the pump and approximately 30 % of those patients were subsequently able to be discharged from the hospital [18].
Medtronic Bio-Medicus Bio-Pump
The Medtronic Bio-Medicus Bio-Pump (Medtronic, Inc, Eden Prairie, MN, USA) is a centrifugal pump. The Bio-Medicus pump is available in most cardiac surgery centers and has been used for femoral-femoral bypass, CPB, VAD, and ECMO [21, 22]. The BPX-80 bio-pump is the version which has seen the most clinical use. The newer generation Affinity bio-pump has been modified to have a lower priming volume, a modified impeller to minimize hemolysis, and two different types of biocompatible surfaces (Fig. 6.7).
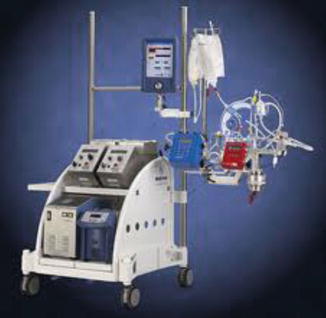

Fig. 6.7
Medtronic Biomedicus pump
The cannula are typically placed either centrally via sternotomy (LVAD, RVAD, or BIVAD) or peripherally via the femoral vessels (LVAD). The cannulation and cannula securing strategies are essentially identical to those employed with CentriMag placement. LVAD inflow cannula into the superior pulmonary vein or left atrium and outflow cannula into the ascending aorta. RVAD inflow cannula is from the right atrium with outflow cannula into the main pulmonary artery.
If the bioactive coatings are used (Carmeda BioActive Surface, Trillium Biosurface, or Balance Biosurface, Medtronics Cardiopulmonary, Anaheim, CA, USA) minimal to no heparinization can theoretically be used. As a practical point, heparinization is often used though however. In the setting where non-heparin bonded lines are utilized, heparin drip systemic heparinization should be used to minimize the risk of thrombosis/thromboembolic events with a goal ACT of 150–200 s. Additional anticoagulation is needed for weaning trials to avoid the risk of clot. When the patient demonstrates an ability to be successfully weaned from temporary MCS, the cannula can be removed and pledgeted purse-strings sutures secured in the standard fashion [21, 22]. This approach is similar to that described for the CentriMag decannulation [8].
The Bio-Medicus pump is available at many cardiac centers in the United States. Due to its duration of service, it is a “tried and true” workhorse biopump of many centers. The use of the Bio-Medicus pump for weaning of post-cardiotomy failure is reported to be 45–70%. The survival of those patients who are able to be weaned from the temporary pump is on the order of 40–60 % [8, 21–24].
Percutaneously Placed Ventricular Assist Devices
Intra-aortic Balloon Pump
When Moulopolous and colleagues published their seminal article on intra-aortic balloon pump (IABP) placement in 1962 [25], it is unlikely that they could have estimated the extent of its adoption. The initial clinical use inpatients was in 1968 by Kantrowitz [26]. The IABP has become the archetype for bedside, percutaneously placed cardiac augmentation.
The IABP is inexpensive, simple to place, portable, and has been effective in treating those patients with cardiogenic shock. The IABP has two functions to improve hemodynamic stability. The first is, through its inflation during diastole, it provides improved coronary arterial perfusion. Secondly, through the synchronized deflation with the cardiac cycle, there is afterload reduction. This afterload reduction has the potential to improve cardiac output. Augmentation should be timed such that deflation occurs immediately prior to aortic valve opening. Insufflation should be timed with aortic valve closure. The timing can be synchronized with the electrocardiogram tracing or with the aortic pressure tracing. An advantage of the IABP is its ease of placement. Through percutaneous access or direct femoral arterial cut-down, the IABP may be placed with a sterile Seldinger technique. Fluoroscopy, plain chest Xray, or trans-esophageal echocardiography are able to be used to visualize correct placement. The proximal tip of the IABP should be place distal to the left subclavian artery. If a protracted duration of IABP use occurs, or if balloon inflation ratios of 1:2 or greater are used, anticoagulation with heparin should be considered.
There are potential complications of IABP use, however. The can be distal limb ischemia. The balloon can rupture and inattention to removal could lead to thromboembolic sequelae. At the entry site, infection, neuropathy, fistula (arteriovenous or lymphatic), pseudo aneurysm formation, aorto-iliac dissection, and arterial rupture. If the IABP is not appropriately placed, there is a potential for mesenteric or renal arterial occlusion.
For decades, IABP has been used as the treatment of choice to initially stabilize patients with cardiogenic shock after myocardial infarction. This has been especially true in the setting of patients who are to undergo revascularization. The role of the IABP in the treatment of cardiogenic shock in the setting of myocardial infarction is being called into question however. In 2012, Theile and colleagues from the IABP-SHOCK II investigators published on a prospective registry of 600 patients who were randomized to IABP or no IABP use, in the setting of patients with myocardial infarction and cardiogenic shock who were expected to undergo early (percutaneous or coronary artery bypass grafting) revascularization. In this study, there was no significant reduction in 30-day mortality, short term outcome metrics, or complications [5].
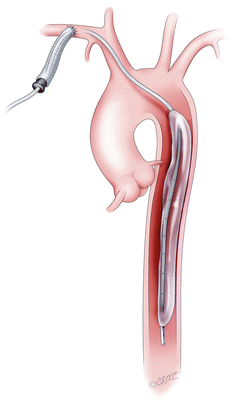
Subclavian IABP
In patients with contra-indications to easy implantation of a conventional LVAD or percutaneous peripheral cannulation, en emerging experience from Chicago with intra-aortic balloon pumps inserted through a graft sewn to the left or right subclavian artery shows great promise [27] (Fig. 6.8).