5 Acute Lung Injury
A wide variety of insults can produce acute lung damage, inclusive of those that injure the lungs directly. Early terms for diffuse acute lung injury occurring indirectly in the setting of overwhelming nonthoracic trauma accompanied by hypovolemia were “shock lung,” “postperfusion lung,” “traumatic wet lung,” and “congestive atelectasis.”1,2
In 1967, Ashbaugh and coworkers formally described a syndrome characterized by acute onset of severe respiratory distress after an identifiable injury. Clinical signs included dyspnea, reduced lung compliance, diffuse chest radiographic infiltrates, and hypoxemia refractory to supplementary oxygen.3 Today this sequence of clinical events is referred to as the acute respiratory distress syndrome (ARDS). The clinical course is rapid and the mortality rate is high, with more than one half of affected patients dying of respiratory failure within days to weeks.2,4,5 A recent meta-regression analysis performed by Zambon and Vincent6 of mortality rates from 72 published studies of ARDS identified a decrease of 1.1% per year for the period 1994 to 2006, with an overall pooled mortality rate for all studies of 43%.
The American-European Consensus Conference (AECC) formally defined ARDS in 1994 using the following criteria: acute onset; bilateral chest radiographic infiltrates; hypoxemia regardless of the positive end-expiratory pressure oxygen concentration, an arterial partial pressure of oxygen to inspired oxygen fraction ratio less than 200, and no evidence of left atrial hypertension.7 The AECC also agreed that ARDS represents the most severe form on a spectrum of disease conditions encompassed under the general term acute lung injury.
The histopathologic counterpart of ARDS is distinctive and referred to as diffuse alveolar damage (DAD). DAD is the most extreme manifestation of lung injury and can occur as a result of a large number of direct injuries to the lungs (e.g., infection). In this chapter, the emphasis is on DAD and less severe manifestations of acute lung injury. Some authors have considered organizing pneumonia to be a form of acute lung injury, but we and others believe that organization is a subacute phenomenon with a more protracted clinical course (extending over several days to weeks). Subacute organizing pneumonia of unknown etiology (previously known as idiopathic bronchiolitis obliterans organizing pneumonia)8 is discussed with the chronic diffuse diseases (see Chapter 7).
Diffuse Alveolar Damage: The Morphologic Prototype of Acute Lung Injury
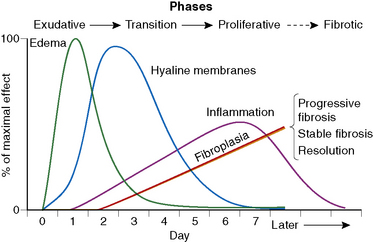
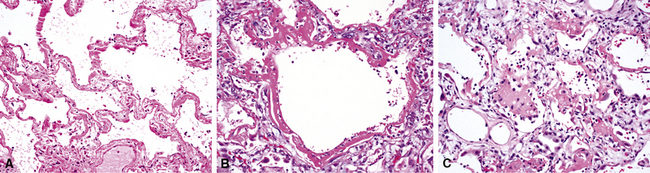
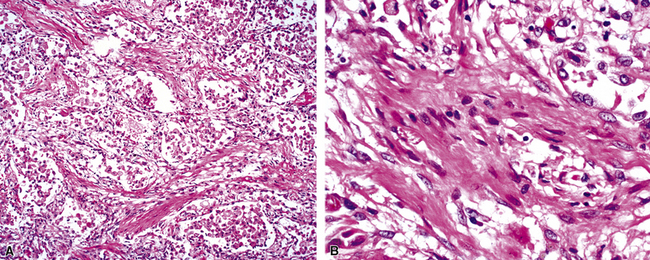
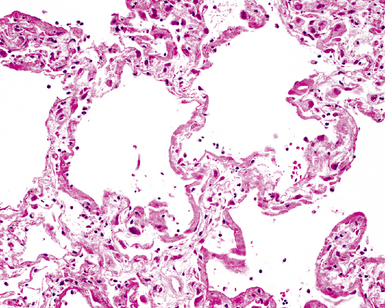
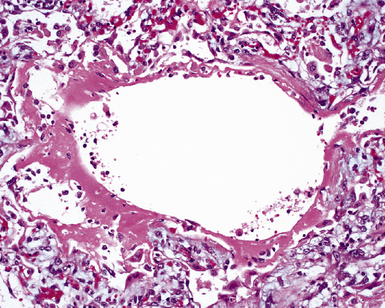
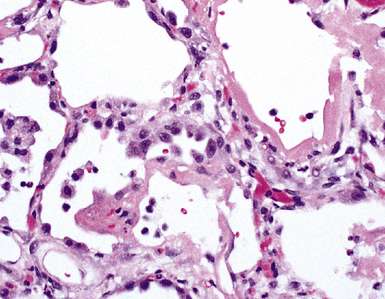
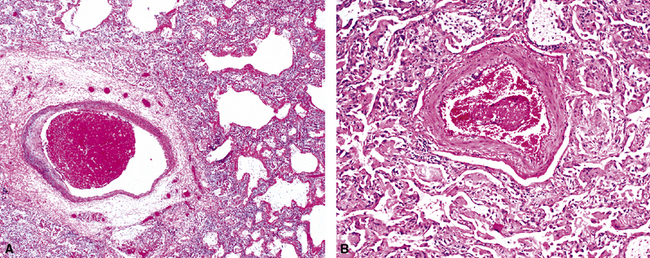
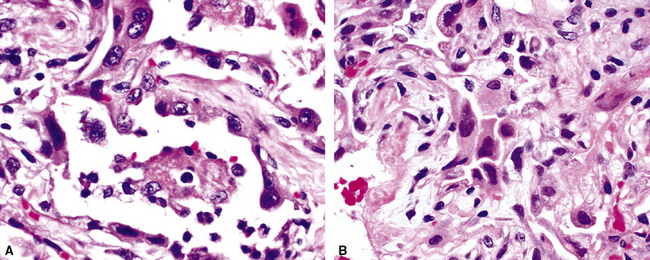
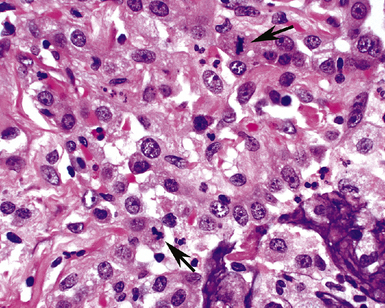
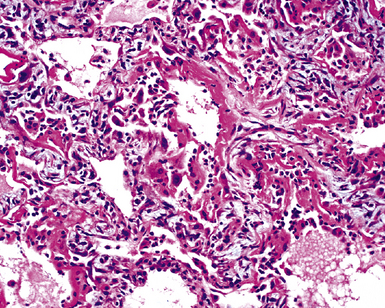
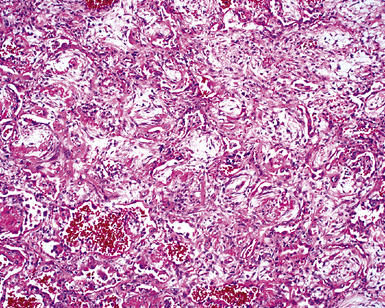
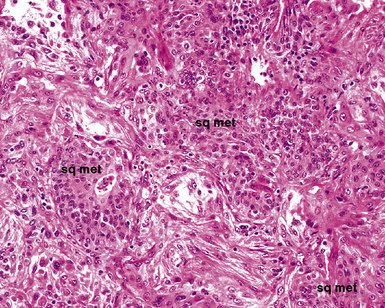
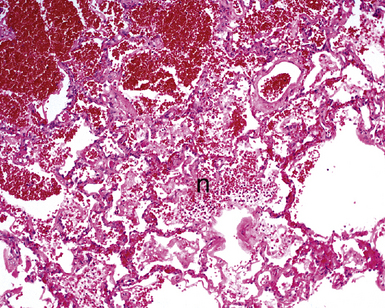
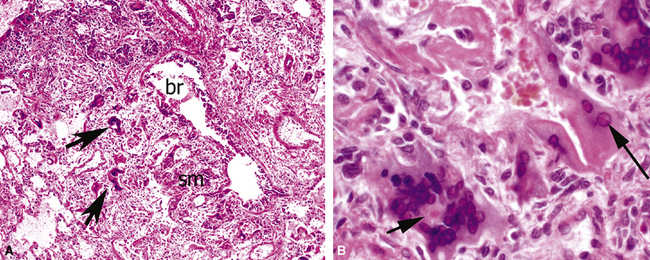
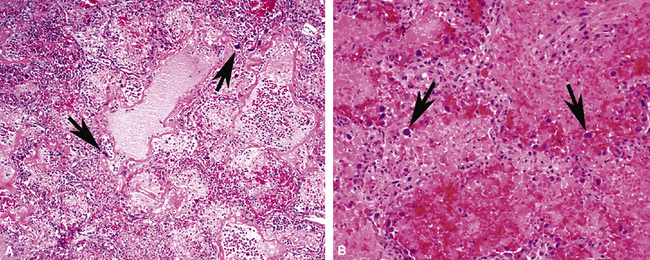
The causes of acute lung injury are numerous (Box 5-1). The lung reacts to various types of insults in similar ways, regardless of etiology. The resultant endothelial and alveolar epithelial cell injury is attended by fluid and cellular exudation. Subsequent reparative fibroblastic proliferation is accompanied by type II pneumocyte hyperplasia.4,9 The microscopic appearance depends on the time interval between insult and biopsy, and on the severity and extent of the injury. 2 DAD is the usual pathologic manifestation of ARDS and is the best-characterized prototype of acute lung injury. From studies of ARDS, the pathologic changes appear to proceed consistently through discrete but overlapping phases (Fig. 5-1)—an early exudative (acute) phase (Fig. 5-2A and B), a subacute proliferative (organizing) phase (see Fig. 5-2C), and a late fibrotic phase (Fig. 5-3).2,4,5,8,10 The exudative phase is most prominent in the first week of injury. The earliest changes include interstitial and intra-alveolar edema with variable amounts of hemorrhage and fibrin deposition (Fig. 5-4). Hyaline membranes (Fig. 5-5), the histologic hallmark of the exudative phase of ARDS, are most prominent at 3 to 7 days after injury. Minimal interstitial mononuclear inflammatory infiltrates (Fig. 5-6) and fibrin thrombi in small pulmonary arteries (Fig. 5-7) also are seen. Type II pneumocyte hyperplasia (Fig. 5-8) begins by the end of this phase and persists through the proliferative phase. The reactive type II pneumocytes may demonstrate marked nuclear atypia, with numerous mitotic figures (Fig. 5-9). The proliferative phase begins at 1 week after the injury and is characterized by fibroblastic proliferation, seen mainly within the interstitium but also focally in the alveolar spaces (Fig. 5-10). The fibrosis consists of loose aggregates of fibroblasts admixed with scattered inflammatory cells (Fig. 5-11); collagen deposition is minimal. Reactive type II pneumocytes persist. Immature squamous metaplasia may occur (Fig. 5-12) in and around terminal bronchioles. The degree of cytologic atypia in this squamous epithelium can be so severe as to mimic malignancy (Fig. 5-13). The hyaline membranes are mostly resorbed by the late proliferative stage, but a few remnants may be observed along alveolar septa. Some cases of DAD resolve completely, with few residual morphologic effects, but in other cases, fibrosis may progress to extensive structural remodeling and honeycomb lung. As might be expected, a recent review of outcomes for 109 survivors of ARDS revealed persistent functional disability at 1 year after discharge from intensive care.11
Box 5-1 Etiology of Diffuse Alveolar Damage
Modified from Katzenstein A, Askin F, eds. Katzenstein and Askin’s Surgical Pathology of Non-Neoplastic Lung Disease, 3rd ed. Philadelphia: Saunders; 1997:16.
By definition, ARDS has a known inciting event. The foregoing description is based on a model of ARDS due to oxygen toxicity, wherein the evolution of histopathologic abnormalities can be studied over a defined time period.2,5 In practice, lung biopsy most often is performed in patients without a known cause or specific time of onset of injury. Moreover, with some causes of acute lung injury, the damage evolves over a protracted period of time, or the lung may be injured in repetitive fashion (e.g., with drug toxicity). In such circumstances, the pathologic changes do not necessarily progress sequentially through defined stages as in ARDS, so both acute and organizing phases may be encountered in the same biopsy specimen. The basic histopathologic elements of acute lung injury are presented in Box 5-2.
Acute fibrinous and organizing pneumonia (AFOP) is a recently recognized histologic pattern of acute lung injury with a clinical presentation similar to that of classic DAD, in terms of both potential etiologic disorders and outcome. It differs from DAD in that hyaline membranes are absent. The dominant feature is intra-alveolar fibrin “balls” or aggregates, typically in a patchy distribution. Organizing pneumonia in the form of luminal loose fibroblastic tissue is present surrounding the fibrin. The alveolar septa adjacent to areas of fibrin deposition show a variety of changes similar to those of DAD, such as septal edema, type II pneumocyte hyperplasia, and acute and chronic inflammatory infiltrates. The intervening lung shows minimal histologic changes. AFOP may represent a fibrinous variant of DAD. In some patients, both DAD and AFOP disease patterns may be present simultaneously.12,13
Specific Causes of Acute Lung Injury
Infection
Infection is one of the most common causes of acute lung injury. Among infectious organisms, viruses most consistently produce DAD.2,5 Occasionally, fungi (e.g., Pneumocystis) and bacteria (e.g., Legionella) also can cause infections manifesting as DAD. Some of the organisms that are well known to cause acute lung injury with characteristic histopathologic changes are discussed next.
Viral Infection
Influenza is a common cause of viral pneumonia. The histopathology ranges from mild organizing acute lung injury (resembling organizing pneumonia) in nonfatal cases to severe DAD with necrotizing tracheobronchitis (Fig. 5-14) in fatal cases.14,15 Specific viral cytopathic effects are not identifiable by light microscopy. On ultrastructural examination, intranuclear fibrillary inclusions may be seen in epithelial and endothelial cells.16
The coronavirus responsible for severe acute respiratory syndrome (SARS) produces the acute lung injury associated with this disorder.17–20 Both DAD and AFOP patterns have been identified in affected patients. On ultrastructural examination, involved lung tissue revealed numerous to moderate numbers of cytoplasmic viral particles in pneumocytes, many within membrane-bound vesicles.21–23 The virus particles were spherical and enveloped, with spike-like projections on the surface and coarse clumps of electron-dense material in the center. Most had sizes ranging from 60 to 95 nm in diameter, but some were as large as 180 nm.
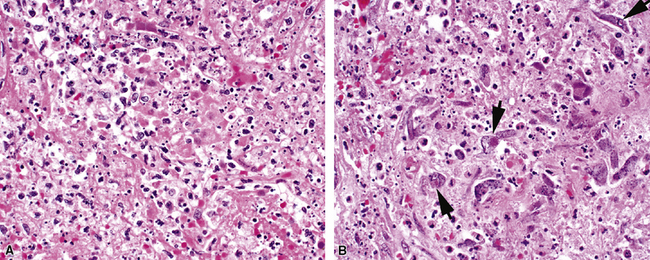
Measles virus produces a mild pneumonia in the normal host but can cause serious pneumonia in immunocompromised children. Histopathologic features of such infection include interstitial pneumonia, bronchitis and bronchiolitis, and DAD.24 The characteristic histologic feature is the presence of multinucleated giant cells (Fig. 5-15A) with characteristic eosinophilic intranuclear and intracytoplasmic inclusions.24–28 These cells are found in the alveolar spaces and within alveolar septa (see Fig. 5-15B). Viral inclusions are seen on ultrastructural examination as tightly packed tubules.28
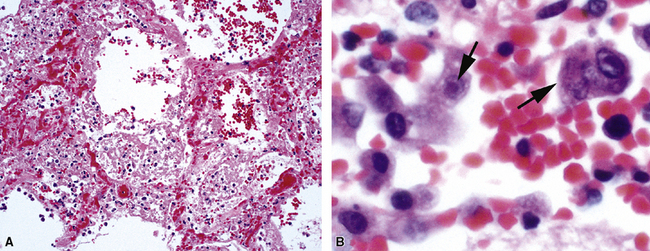
Adenovirus is an important cause of lower respiratory tract disease in children,29,30 although adults (particularly those who are immunocompromised)31 and military recruits also are occasionally affected.32 The lung shows necrotizing bronchitis, or bronchiolitis, accompanied by DAD. The pathologic changes are more severe in bronchi, bronchioles, and peribronchiolar regions (Fig. 5-16A). Two types of inclusions can be observed in lung epithelial cells: An eosinophilic intranuclear inclusion with a halo usually is less conspicuous than the more readily identifiable “smudge cells” (see Fig. 5-16B). These latter cells are larger than normal and entirely basophilic, with no defined inclusion or halo evident by light microscopy.29 On ultrastructural examination, smudge cell inclusions are represented by arrays of hexagonal particles.33
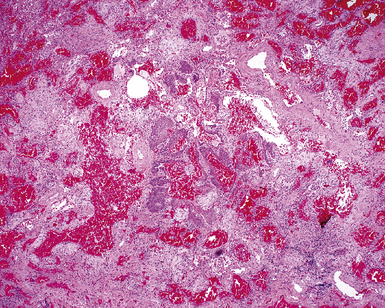
Herpes simplex virus is mainly a cause of respiratory infection in the immunocompromised host. Two patterns of infection are recognized: airway spread resulting in necrotizing tracheobronchitis (Fig. 5-17) and blood-borne dissemination producing miliary necrotic parenchymal nodules. DAD and hemorrhage can occur in both forms.34,35 Characteristic inclusions may be seen in bronchial and alveolar epithelial cells (Fig. 5-18). The more obvious type is an intranuclear eosinophilic inclusion surrounded by clear halo (Cowdry A inclusion), and the other is represented by a basophilic to amphophilic ground-glass nucleus (Cowdry B inclusion). Rounded viral particles with double membranes are seen under the electron microscope.34,35
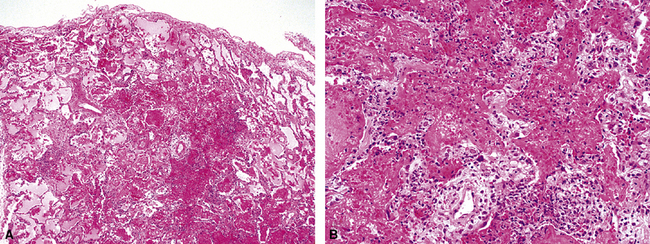
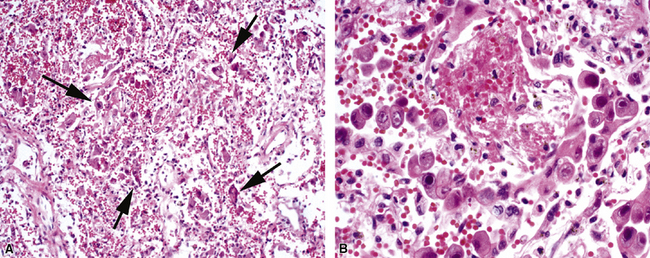
Figure 5-17 Diffuse alveolar damage in herpes simplex pneumonia. Herpesviridae viruses are capable of producing nodular necrotizing pneumonia (see Chapter 6). A, The nodular appearance of lung involved by herpes simplex pneumonia is evident, with zonal areas of hemorrhage and necrosis. B, A higher-magnification view of the hemorrhagic and necrotizing pneumonia.
Varicella-zoster virus causes disease predominantly in children and is the agent of chickenpox.36 Pulmonary complications of chickenpox are rare in children with normal immunity (accounting for less than 1% of the cases). By contrast, however, pneumonia develops in 15% of adults with chickenpox; immunocompetent and immunocompromised persons are equally affected.32,36 The histopathologic picture in varicella pneumonia (Fig. 5-19) is similar to that in herpes simplex. Although identical intranuclear inclusions are reported to occur,32,36 these can be considerably more difficult to identify in chickenpox pneumonia.
Cytomegalovirus is an important cause of symptomatic pneumonia in immunocompromised persons, especially those who have received bone marrow or solid organ transplants, and in patients with human immunodeficiency virus (HIV) infection.37–39 The histopathologic findings range from little or no inflammatory response to hemorrhagic nodules with necrosis (Fig. 5-20A) and DAD.37 The diagnostic histopathologic pattern, seen in endothelial cells, macrophages, and epithelial cells, consists of cellular enlargement, a prominent intranuclear inclusion, and an intracytoplasmic basophilic inclusion37 (see Fig. 5-20B).
Hantavirus is a rare cause of acute lung injury.40–42 The infection produces alveolar edema, hyaline membranes, and atypical interstitial mononuclear inflammatory infiltrates40–42 (Fig. 5-21). Spherical membrane-bound viral particles have been found in the cytoplasm of endothelial cells by electron microscopy.
Fungal Infection
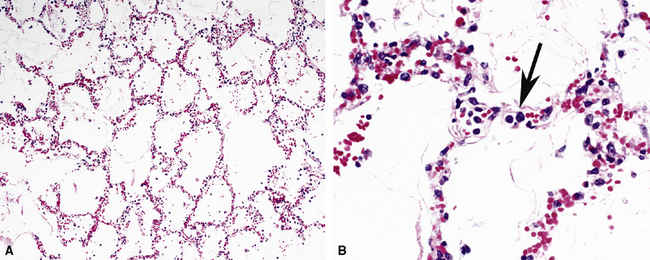
Pneumocystis jiroveci (previously known as Pneumocystis carinii) is the most common fungus to cause DAD.43–45 The histopathology of Pneumocystis infection in the setting of profound immunodeficiency is one of frothy intra-alveolar exudates (Fig. 5-22A) (so-called “alveolar casts”), with many organisms44,45 (see Fig. 5-22B). In the mildly immunocompromised patient, however, this feature is not observed, or the pathologic changes may be subtle. In such cases, several “atypical” manifestations have been described.43,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree