Chapter 31 Acute lung injury
 Acute lung injury is lung inflammation that develops in response to a variety of both pulmonary and generalised acute diseases.
Acute lung injury is lung inflammation that develops in response to a variety of both pulmonary and generalised acute diseases. The clinical features of acute lung injury vary from mild, self-limiting dyspnoea to rapidly progressive and fatal respiratory failure.
The clinical features of acute lung injury vary from mild, self-limiting dyspnoea to rapidly progressive and fatal respiratory failure. Widespread pulmonary inflammation causes increased permeability of the alveolar capillary membrane leading to flooding and collapse of alveoli and severely impaired gas exchange.
Widespread pulmonary inflammation causes increased permeability of the alveolar capillary membrane leading to flooding and collapse of alveoli and severely impaired gas exchange.Acute lung injury (ALI) describes a characteristic form of parenchymal lung disease, and represents a wide range of severity from short-lived dyspnoea to a rapidly terminal failure of the respiratory system, when the term acute respiratory distress (ARDS) is normally used. The syndrome was first described in 1967 when Ashbaugh et al1 reported a condition in adults that seemed similar to the respiratory distress syndrome seen in infants. One of the subjects reported in this original paper was aged only 11 years, and in recognition of the fact that respiratory distress syndrome is known to occur in children the term adult respiratory distress syndrome should be avoided. There are a great many other synonyms for ALI, including acute respiratory failure, shock lung, respirator lung, pump lung and Da Nang lung.
Clinical Aspects of Acute Lung Injury2,3,4
Definition
There is no single diagnostic test, and confusion has arisen in the past from differing diagnostic criteria. This has complicated comparisons of incidence, mortality, aetiology and efficacy of therapy in different centres. To address this problem, European–American consensus conferences produced the following widely accepted definitions.5
Acute lung injury diagnosis requires the presence of four criteria:
These definitions are now widely accepted and have been extremely helpful in researching ALI, particularly epidemiological studies. However, there are several provisos to their use in the clinical situation. For example, it is possible for patients with diseases that elevate left atrial pressure to also have ALI, but they would fall outside the strict definition. Also, many earlier definitions suggest that one or more of the known predisposing conditions should have been present and that the clinical course has followed the recognised pattern (see below). Finally, it is noted that the histology is usually diagnostic but it is seldom indicated or advisable to take a lung biopsy. There is no reliable laboratory test to confirm the diagnosis (see below).
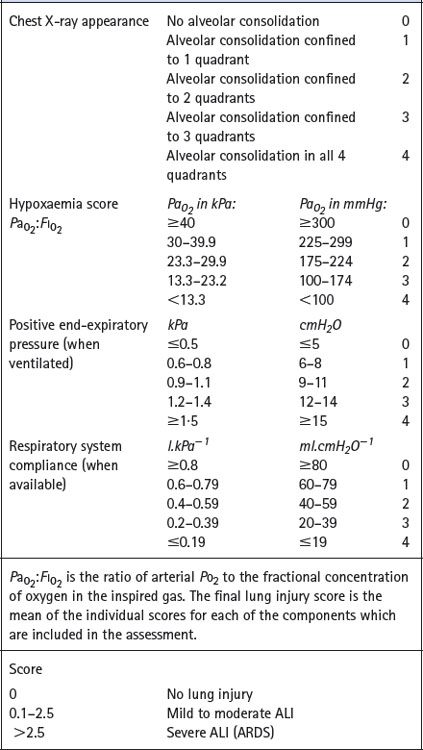
Scoring systems. Various attempts have been made to derive a single numerical value to assess the severity of ALI. Murray et al6 proposed an expanded three-part definition comprising distinction between acute and chronic phases, identification of aetiological and associated conditions and a numerical lung injury score, details of which are shown in Table 31.1.
Predisposing Conditions and Risk Factors for ALI
Although the clinical and histopathological pictures of ALI are remarkably consistent, they have been described as the sequel to a large range of predisposing conditions (Table 31.2). There are, however, very important differences in the progression of ALI and its response to treatment, depending on the underlying cause and associated pathology. Nevertheless, recognition of the predisposing conditions is crucially important for predicting which patients are at risk and the establishment of early diagnosis.
Table 31.2 Some predisposing conditions for ALI
| DIRECT LUNG INJURY | INDIRECT LUNG INJURY |
|---|---|
| Common: Pneumonia Aspiration of gastric contents | Common: Sepsis Severe non-thoracic trauma Multiple transfusions of blood-products |
| Less common: Lung contusion Near drowning Inhalation of toxic gases or vapours Fat or amniotic fluid embolus Reperfusion oedema, e.g. following lung transplantation | Less common: Acute pancreatitis Cardiopulmonary bypass Severe burns Drug overdose Disseminated intravascular coagulation |
The conditions listed in Table 31.2 are not equally likely to proceed to ALI. Studies have consistently identified sepsis syndrome (see below) as the condition most likely to result in development of ALI with about 40% of patients being affected.7 Patients who have aspirated gastric contents, received multiple emergency transfusions or incurred pulmonary contusions have a 17–24% chance of developing ALI. Overall, 25% of patients with a single risk factor develop ARDS but this rises to 42% with two factors and 85% with three. Age and sex do not affect the likelihood of developing ALI.
Pulmonary and extrapulmonary ALI. Gattinoni et al8 proposed that patients with ALI should be considered as two separate groups. Pulmonary ALI results from clinical conditions that cause direct lung injury, whilst extrapulmonary ALI follows indirect lung injury (Table 31.2). These two sub-groups of ALI have been shown to differ with respect to pathological mechanisms, appearances on chest radiographs and CT scans, abnormalities of respiratory mechanics and response to ventilatory strategies.9
Incidence and Mortality10
In the past, the lack of accepted definitions of lung injury led to widely varying estimates of the incidences of ALI and ARDS. Despite more consistent diagnostic criteria in recent years, the estimated incidence of ALI remains variable at 18–79 cases per year per 100 000 population.11 The reasons for this variation in estimates of the incidence of ALI is unknown.2 Around 70% of cases of ALI are severe enough to be classified as ARDS.
There is, however, considerable agreement that mortality from ALI is high: two decades ago in excess of 50% of patients died whatever the criteria of diagnosis. Outcome has improved in recent years with current estimates of around a 40% mortality rate, but still with wide variability,3,12 and only slow progress.13 There appears to be no difference in the mortality of ALI arising from pulmonary or extrapulmonary causes.14
Clinical Course
It is only in phase three that the diagnostic criteria of ALI become established. There is significant arterial hypoxaemia due to an increased alveolar/arterial Po2 gradient, and the arterial Pco2 may be slightly elevated. The lungs become stiff and the chest radiograph shows the characteristic bilateral diffuse infiltrates. Ventilatory support is usually instituted at this stage, but many patients escape this intervention and are managed on respiratory wards rather than in intensive care.15
Pathophysiology
Alveolar/capillary permeability is increased substantially throughout the course of ALI.16 This may be demonstrated by the enhanced transit of various tracer molecules across the alveolar/capillary membrane (page 425).17
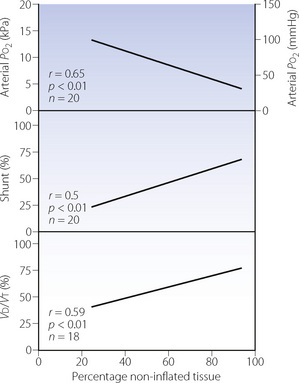
Maldistribution of ventilation and perfusion.18 Computerised tomography (CT) of patients with ARDS shows that opacities representing collapsed areas are distributed throughout the lungs in a heterogeneous manner but predominantly in the dependent regions.19 Following a change in posture, the opacities move to the newly dependent zones within a few minutes.20 The most conspicuous functional disability is the shunt (Figure 31.1), which is usually so large (often more than 40%) that increasing the inspired oxygen concentration cannot produce a normal arterial Po2 (see the isoshunt chart, Figure 8.11). CT scans of patients with ALI also demonstrate substantial areas of lung overdistension.21 These areas contribute to the increased dead space, which may exceed 70% of tidal volume and requires a large increase in minute volume to attempt to preserve a normal arterial Pco2. Both shunt and dead space correlate strongly with the non-inflated lung tissue seen with CT (Figure 31.1).
Lung mechanics. In established ARDS, lung compliance is greatly reduced and the static compliance of the respiratory system (lungs + chest wall) is of the order of 300 ml.kPa−1 (30 ml.cmH2O−1).22 Patients with pulmonary and extrapulmonary forms of ARDS (see above) have different abnormalities of respiratory system mechanics.8 Respiratory system compliance is reduced to a similar extent in both groups, but the abnormality is mostly with lung compliance when lung disease is the cause, and chest wall compliance with extrapulmonary causation.
Functional residual capacity is reduced by collapse and increased elastic recoil.
Mean total resistance to airflow was found to be 1.5–2 kPa.l−1.s (15–20 cmH2O.l−1.s),22 or about three times that of anaesthetised patients with normal lungs, measured by the same technique. Using the model shown in Figure 4.4, some two-thirds of the total resistance in patients with ARDS could be assigned to visco-elastic resistance of tissue, although the airway resistance was still about twice normal.
Oxygen consumption by the lung. Pulmonary oxygen consumption (page 212) can be very high in patients with severe ALI. It is possible that some of this represents reactive oxygen species formation (see Chapter 26), but the increase in pulmonary oxygen consumption does not seem to correlate with various markers of pulmonary inflammation at the time the measurement is made.23
Mechanisms of Acute Lung Injury
Histopathology
Although of diverse aetiology, the histological appearances of ARDS are remarkably consistent and this lends support for ARDS being considered a discrete clinical entity. Histological changes at autopsy may be divided into two stages, as follows.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree