Fig. 19.1
(a) Sinus tachycardia with poor R-wave progression in the precordial leads, suggesting old anterior myocardial infarction. There is ST depression in the leads I, II, III, aVF, and V6. Therefore, there is reciprocal ST elevation in lead aVR. There is minimal ST elevation in lead V1, and the T wave in lead V2 is tall and positive, suggesting ischemia (Grade 1 of myocardial ischemia). (b) Several hours later, after resolution of symptoms, there is less ST depression in the inferior and lateral leads. ST elevation in leads aVR and V1 resolved, and the T-wave amplitude in V2 decreased. In addition, the T waves in aVL are now negative, suggestive of resolution of ischemia. (c) The next day, a QS wave is seen in lead V2, and there is now ST elevation in leads V2–V3, suggestive of an anterior myocardial infarction
ST elevation is the most dramatic ECG manifestation of acute transmural myocardial ischemia in the leads facing the ischemic zone. In the leads facing to the anatomically opposite myocardial segments, reciprocal ST depressions can be detected. These ST depressions do not represent additional subendocardial ischemia, but rather is a pure electrical phenomenon of reciprocal changes. Based on the distribution and morphology of ST changes in the ECG leads and the presence of coexistent QRS- or T-wave changes, different ECG classifications were developed. These are regarded as markers of severity of myocardial ischemia and extent of the ischemic process; they also reflect temporal differences during the disease process.
When ischemia is confined primarily to the subendocardium, the overall ST vector typically faces the inner ventricular layer and the ventricular cavity such that the surface ECG leads show ST depression [11]. This subendocardial ischemic pattern is a frequent finding during spontaneous episodes of rest angina such as appears during ischemic imbalance. In severe extensive subendocardial ischemia, as in acute subtotal or even total occlusion of the left main coronary artery, the ECG shows ST depression in the majority of the ECG leads and ST elevation in lead aVR [12].
19.4 Historical Aspects of ACS Classification
The association between abnormally wide Q waves and MI was established by historical works in the 1930s–1940s [13, 14]. Originally, Q-wave MI was considered strongly associated with transmural infarction. Later on, moderate sensitivity and good specificity for Q waves to detect myocardial necrosis at autopsy in patients with a narrow QRS complex was reported [15]. Adverse post-MI outcome was evident from studies conducted before the introduction of reperfusion therapy – fibrinolysis or PCI. Q-wave duration, Q/R ratio, and Q waves in multiple infarct locations were associated with worse long-term outcome [16].
In the chronic phase of MI, Q waves are regarded as a sign of irreversible necrosis. However, about 50 % of patients presenting within 1 h of onset of ST-elevation acute coronary syndrome (STE-ACS) already have Q waves in the leads with ST elevations, especially in the anterior leads. These Q waves may be transient and not necessarily represent irreversible damage. It is believed that intense ischemia may cause a transient loss of electrical activity in the region at risk. Thus, Q waves on presentation may reflect either irreversible damage and/or a large ischemic zone and thus portend a large final infarction. On the other hand, in inferior STE-ACS, preexisting Q waves may disappear during acute ischemia – the Q waves may be “pulled up” by the injury current – and reappear during reperfusion.
The traditional view that Q waves represent transmural MI, while subendocardial MI is reflected by ECG changes other than Q waves, was questioned in the 1980s [17]. It was evident from autopsy studies that about half of subendocardial MIs caused pathological Q waves, while about half of transmural MIs did not [18]. Accordingly, the terms Q-wave and non-Q-wave MI replaced the former designations. The most recent golden standard of myocardial injury, cardiac magnetic resonance imaging (CMR), has shown that the Q-wave/non-Q-wave distinction is determined by the total size rather than by the transmural extent of the underlying scar [19].
Braunwald’s group introduced the concept of reduction of infarct size by pharmacologic agents in the late 1960s–1970s, and that resulted in a need for refinement of the classification of acute coronary events [20]. The term unstable angina (acute ischemia without resultant detectable necrosis) was introduced in the early 1970s and was classified according to severity by Braunwald in the late 1980s [21, 22].
19.5 ACS Classification of MI Based on Location
After the introduction of the three original ECG leads, I, II and III, by Einthoven in 1912, investigators began to classify MIs based on changes in the QRS complex according to their location into anteroapical and posterobasal MI. With the development of the unipolar leads and multiple chest lead technique, additional locations were introduced. Monumental work with systematic autopsy studies in the 1930s and 1940s established the theoretical and empirical basis for the association between different ECG patterns and the location of myocardial necrosis [14]. Based on pathological correlation, a relationship between the location of infarcted areas and Q waves on the ECG was, until recently, accepted and, with minor modifications, implemented in scientific statements and textbooks.
Already in the 1930s, studies showed different premortal ECG patterns depending on the MI location at autopsy. In patients, who survived the acute infarction, coronary angiography enabled the correlation of ECG signs of myocardial necrosis with this new technique. Reperfusion therapy, initially by intracoronary thrombolysis in the 1980s, resulted in a clear need to improve the ECG diagnosis to be able to predict the culprit artery and the estimated size of the area at risk by observing changes from the acute occlusive phase (ST/T changes). Since then, many ECG algorithms for the prediction of the culprit artery and the level of vessel occlusion have been introduced. The results of the studies evaluating these algorithms vary; they are dependent on factors such as time from ECG recording to angiography, individual variation of coronary artery anatomy, and coronary artery dominance.
More recently, the correlation between Q waves in various ECG leads and the affected myocardium has been studied by CMR. Based on these findings, a new terminology for LV walls and location of Q-wave MIs was proposed [23]. The consensus group recommended to classify the different MI locations based on the following six most commonly occurring patterns of abnormal Q waves and Q-wave equivalents (Fig. 19.2). In septal MI, the septal wall and often a small part of the adjacent anterior wall are involved, and the ECG shows Q waves in leads V1 and V2 [24]. The term mid-anterior was recommended for MIs located especially in the mid-low segments of the anterior wall. This MI subtype is typically caused by occlusion of the first diagonal branch, and in the ECG Q waves are present in leads aVL (I) and sometimes V2. Compared with septal infarction, in apical-anterior MI, the abnormal Q waves extend into the more leftward precordial leads – typically V3 and V4 and sometimes V5 and V6. There are no abnormal Q waves in leads aVL and I. Extensive anterior infarction is essentially a combination of the three previously mentioned types. Consequently, the ECG shows abnormal Q waves in the precordial leads and lead aVL (sometimes also in lead I). In lateral MI, the ECG may produce the Q-wave equivalents of abnormally prominent R waves in leads V1 and V2. There may also be abnormal Q waves in lead I, aVL, and/or V5 and V6. Finally, inferior MIs produce Q waves in leads II, III, and aVF, but without increased R waves in leads V1 and V2. When the infarct-related artery (right coronary or left circumflex artery) is very dominant and the occlusion is proximal, the infarction encompasses both the inferior and the lateral wall, and then the ECG pattern is the association of criteria of inferior and lateral MI (inferolateral MI).
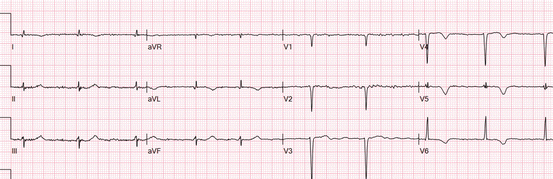

Fig. 19.2
Old anterior myocardial infarction with Q-wave equivalent small R waves in the leads V2–V5 and Q waves in the leads aVL and V1. There are no signs of acute myocardial ischemia; there are no ST elevations, and the T waves are inverted in the leads I, aVL, and V4–V6 (postischemic changes)
19.5.1 EGG Coding and Scoring Systems
The Minnesota Code was developed in the late 1950s in response to the need for reporting ECG findings in objective, uniform, and clearly defined terms [25]. In clinical trials and epidemiologic studies, this coding system is the most widely used ECG classification system. Changes in the QRS complex, the ST segment, and the T wave are included in the system; these ECG parameters are typically altered in ACS. With the introduction of the concept of myocardial salvage by Braunwald’s group in the 1960s and 1970s, there was a need for universally available diagnostic methods to assess infarct size [20].
19.5.1.1 Estimates of the Extent of Myocardial Ischemia/Infarction
In the mid-1980s, the Selvester QRS scoring system was developed for the estimation of the total percentage of the left ventricle that is infarcted by using a weighted scoring system [26]. Originally, studies confirmed the agreement between the Selvester QRS score and MI size determined by postmortem histopathology in patients with non-reperfused MI. More recently, the agreement with CMR-determined MI size in reperfused STEMI has proven to be poor [27].
Later on, the Aldrich score was developed for estimating the extent of myocardium at risk for infarction by quantitating initial ST changes on the presenting ECG [28]. This score is expressed as % myocardium at risk of infarction. The Aldrich ST score has been shown to underestimate myocardium at risk when compared with single-photon emission computed tomography (SPECT) imaging [29]. In a recent study, the score did not provide a stable estimate of myocardium at risk between prehospital and hospital ECGs [30]. Theoretically, an ECG score to estimate area at risk should include quantitative measurements of both the ST-segment and QRS-complex abnormalities.
It is commonly accepted that the absolute amplitude of ST deviation and/or extent of ST deviation (as reflected in the number of leads with threshold ST elevation) correlates with the size of the myocardial injury. It has been suggested that the magnitude of ST elevation in the inferior leads in inferior STEMI and the number of leads with ST elevation in the precordial leads in anterior STEMI (Aldrich score) correlate with the predischarge Selvester QRS score [31]. However, the correlation between the Aldrich score and the size of the ischemic area at risk (assessed by pretreatment technetium Tc 99 m sestamibi scan or CMR) in patients undergoing reperfusion therapy was weak. The existence of opposite ischemic vectors with cancellation and attenuation of ST deviations could be a plausible explanation for the weak correlation. Another possible explanation for the attenuation of ST elevation is preconditioning by either ischemia or pharmacological agents.
19.5.1.2 Estimation of Acuteness
In animal models, the amount of irreversibly injured myocardium increases with longer duration of ischemia, whereby the extent of salvageable myocardium logically decreases. Due to the dynamic nature of thrombosis formation and spontaneous fibrinolysis with resultant varying degrees of coronary flow, exact ischemic time (time from occlusion to reperfusion of the infarct-related artery) is often difficult to establish in clinical settings. Nevertheless, time from symptom onset to reperfusion (treatment delay) has been used as a proxy for total ischemic time [32]. This time interval is divided into different fragments, such as time from first medical contact to balloon inflation, door-to-balloon time, etc., and are used as measures of assurance for quality standards in health care and also as outcome predictors. Controversy exists regarding the ability of time measures to predict myocardial salvage, final infarct size, and mortality, all of which are important prognostic factors.
Anderson-Wilkins and colleagues developed an ECG acuteness score to augment historical timing of the acute symptom onset or, alternatively, to estimate viability [33]. This score is provided as a continuous scale from 4.0 (hyperacute) to 1.0 (subacute) based on the comparative hyperacute T waves versus abnormal Q waves in each of the leads with ST elevation. However, the independent significance of the T-wave amplitude apart from the presence of Q waves has not been tested. The ECG method of acuteness score was superior to historical timing in predicting myocardial salvage and prognosis after reperfusion therapy, suggesting that ECG-estimated duration of ischemia might provide a better and objective means to select acute reperfusion therapy rather than the subjective patient history, which could potentially preclude proper reperfusion in some patients with salvageable myocardium presenting late [34]. In general, it could be questioned, whether these ECG parameters reflect timing or if it would be more appropriate to refer to them as an estimate of viability, as the rate of progression of myocardial necrosis over time varies considerably.
Sclarovsky introduced a simple method, without the need for counting scores, to estimate acuteness of the infarct process in STEMI [35]. In this classification, ST elevation without T-wave inversions or Q waves is defined as the “preinfarction syndrome” (Fig. 19.3), while ST elevations accompanied by inverted T waves and/or Q waves are referred to as evolving MI (Fig. 19.4). The hypothesis is that the preinfarction syndrome represents the window of opportunity for reperfusion therapy, while evolving MI indicates a later stage of the process with irreversible myocardial injury. Preliminary, retrospective data showed differences in outcome in STEMI patients depending on this classification [36]. Especially in patients with anterior STEMI and evolving MI without reperfusion (no inverted T waves), primary PCI was superior to fibrinolysis.
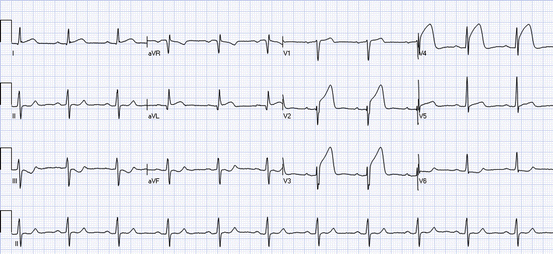
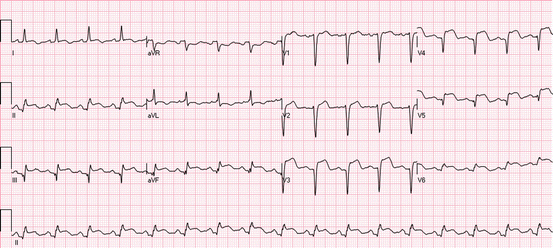

Fig. 19.3
Anterior ST elevations: the ECG shows ST elevations in leads I, aVL, and V1–V5. Reciprocal ST depressions are present in leads III, aVF, and V6. Preinfarction syndrome: ST elevations without Q waves or inverted T waves in the leads with ST elevations. Grade 2 ischemia: ST elevations without changes in the QRS complex

Fig. 19.4
Anterior and inferior ST elevations: the ECG shows ST elevations in leads I, II, III, aVF, and V1–V6. Evolving MI: Q waves are present in leads V3–V5, and there are minor terminal T-wave inversions in V4–V6
19.5.1.3 Grade of Ischemia
Shortly following an occlusion of an epicardial coronary artery, the T waves become positive, tall, and symmetrical in leads with their positive poles facing the ischemic zone, and later on ST elevations develop. In some patients, changes in the terminal portion of the QRS may also be detected if myocardial ischemia continues. Myocardial protection has been proposed as the underlying physiological factor in this classification system, named the Sclarovsky-Birnbaum grading of ischemia [10]. According to the prevailing hypothesis, the changes in the terminal portion of the QRS complex reflect severe ischemia and are believed to be caused by prolongation of the electrical conduction in either the Purkinje fibers or the myocardium in the ischemic zone. For practical purposes, Sclarovsky categorized these changes into three grades of ischemia: Grade 1, tall symmetrical T waves without STE (Fig. 19.5); Grade 2, STE with tall T waves without terminal QRS distortion (Fig. 19.3); and Grade 3, STE with positive T waves with distortion of the terminal portion of the QRS (disappearance of the S waves below the isoelectric lines in leads with Rs configuration (usually V1–V3) or emergence of the J-point >50 % of the R waves in leads with qR configuration) (Fig. 19.6).
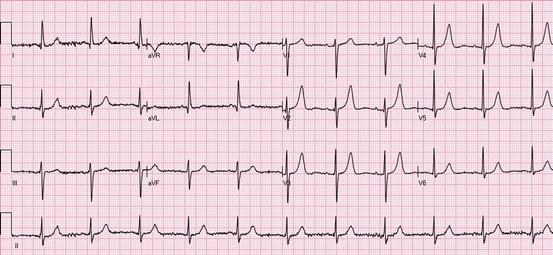
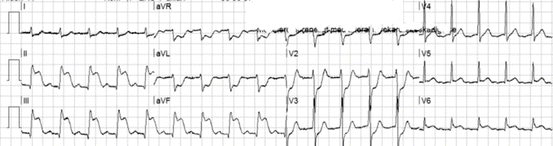

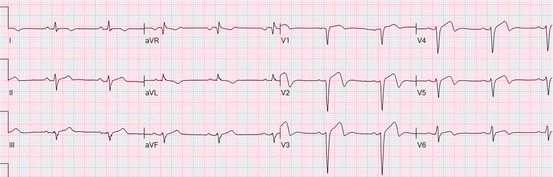
Fig 19.5
Tall T waves in the precordial leads represent Grade 1 ischemia

Fig. 19.6
Inferior and lateral ST elevations: the ECG shows ST elevations in the leads II, III, aVF, and V5–V6 and reciprocal ST depressions in I, aVR, aVL, and V1–V4. Grade 3 ischemia: the J-point is elevated >50 % of the height of the R wave in leads II, III, and aVF
A small percentage of patients presenting with ongoing chest pain due to coronary artery occlusion present with only Grade 1 of ischemia (tall T waves without ST elevation) [37]. Continuous ECG recording showed that without reperfusion therapy, these patients may develop Q-wave MI in the subsequent days. The patients tend to have collateral circulation to the infarcted zone, probably secondary to preexisting subtotal occlusion of the artery, suggesting partial protection by ischemic preconditioning or residual perfusion via the collateral circulation.
Studies performed by several independent groups clearly showed that a Grade 3 ischemia pattern on the presenting ECG is associated with larger final infarct size, less myocardial salvage, and poorer clinical outcomes, especially in patients presenting relatively late (>2–3 h after onset of symptoms), despite prompt reperfusion by fibrinolysis or primary PCI [38]. The Sclarovsky-Birnbaum score is an empiric practical tool to categorize ECG findings for better risk assessment. However, no prospective studies randomizing patients to different treatment groups based on the grade of ischemia have been published. Hence, we have no data to support different therapeutic strategies in patients with Grade 3 compared with Grade 2 ischemia, although patients with the former have worse outcome.
19.5.2 Standard ECG Criteria in the Diagnosis of Myocardial Infarction
The World Health Organization (WHO) has played a leading role in the formulation and application of standard criteria for the diagnosis of MI. The WHO Expert Committee in 1959 established the ECG criteria for “very probable” MI, which was mainly based on Q waves with concomitant T-wave changes [39]. In the Joint International Society and Federation of Cardiology/World Health Organization Task Force statement of 1979, unequivocal ECG findings were the development of abnormal, persistent Q or QS waves and evolving “injury current” lasting longer than 1 day [40]. The joint ESC/ACC committee consensus document published in 2000 highlighted the need for classification into STEMI and NSTEMI instead of Q-wave and non-Q-wave MI in the acute phase, mainly because of the importance of reperfusion therapy in STEMI [2].
The Universal definition of MI, published in 2007, introduced different cut points for ST elevation in men and women in leads V2–V3 [41]. Population studies have clearly shown gender differences especially in these leads. In the most recent universal definition of MI, an additional factor – patient age – was introduced [5]. Importantly, the authors pointed out that lesser degrees of ST displacement or T-wave inversion do not exclude acute myocardial ischemia or evolving MI, since a single static recording may miss the more dynamic ECG changes that might be detected with serial recordings. They also recommended to use supplemental leads such as V3R and V4R (reflecting the free wall of the right ventricle) and V7–V9 (reflecting the basal inferolateral wall), as well as serial ECG recordings, in patients who present with ischemic chest pain and a nondiagnostic initial ECG.
19.5.2.1 Proposal for a New ECG-Based Classification of ACS
A recent working group statement questioned the definitions in the prevailing ECG classification of STE- and non-STE-ACS [12]. The statement recommended a classification based on the pathophysiologic/electrophysiologic processes involved instead of strict classification based on the ECG findings per se. The time point of patient presentation and the recording of the ECG with relation to the pathological processes taking place in the coronary artery and the myocardium is a critical aspect determining the classification of ACS. If the presenting ECG is recorded during the acute occlusive stage, ST elevations are present in the ECG leads overlying the ischemic area. Then the initial diagnosis will be STE-ACS. However, if spontaneous fibrinolysis is effective or if the patient has received antithrombotic medication and the coronary artery has opened before the initial ECG is recorded, inverted T waves without ST elevations may be present. This will result in an initial diagnosis of NSTE-ACS (Fig. 19.7). The classification does not state that all ECG patterns classified as STE-ACS should necessarily indicate the need for emergent reperfusion therapy. Instead, by adding pathophysiological aspects into the ECG classification, the authors wanted to correct some prevailing misunderstandings. One of these is related to the underlying mechanisms of inverted T waves without ST depression in ACS (see below).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


