Chapter 5 Acute Coronary Syndromes
Upon completion of this chapter, you will be able to:
Introduction
In 2010, about 785,000 Americans experienced a new heart attack (MI) and about 470,000 had a recurrent attack. An estimated additional 195,000 silent heart attacks occur each year. The average age of an individual having a first heart attack is 64.5 years for men and 70.3 years for women.1
Pathophysiology of Acute Coronary Syndromes
[Objective 1]
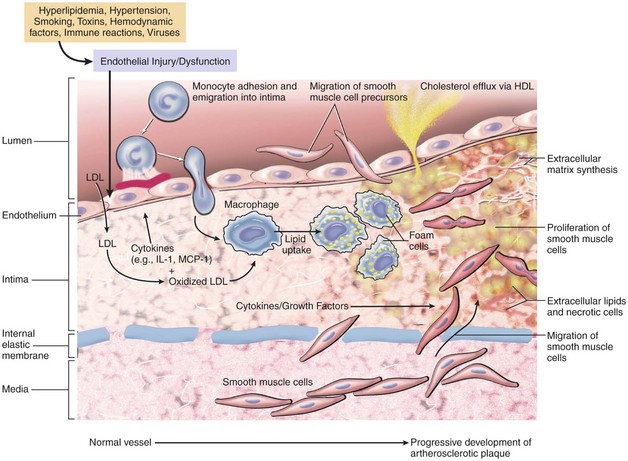
Research has shown that oxidation and the body’s inflammatory response contribute to atherosclerosis and heart disease. A hypothetical sequence of cellular interactions in atherosclerosis is shown in Figure 5-1. Oxidation is a normal chemical process in the body that is caused by the release of free radicals. Free radicals are oxygen atoms created during normal cell metabolism. Too many free radicals can seriously damage cells and impair the body’s ability to fight against illness. Examples of conditions that can cause an overproduction of free radicals include stress and exposure to cigarette smoke, pesticides, air pollution, ultraviolet light, and radiation.
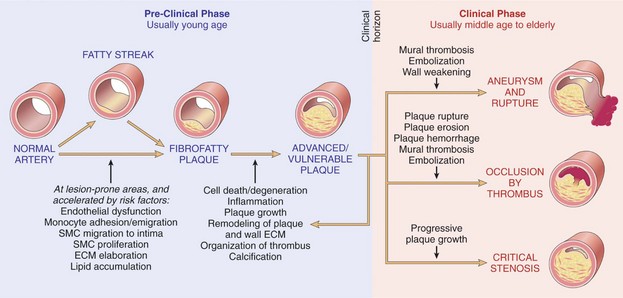
Atherosclerotic lesions include the fatty streak, the fibrous plaque, and the advanced (complicated) lesion (Figures 5-2 and 5-3). Fatty streaks are thin, flat yellow lesions composed of lipids (mostly cholesterol) or smooth muscle cells that protrude slightly into the arterial opening. They appear in all populations, even those with a low incidence of coronary artery disease (CAD). Fatty streaks do not obstruct the vessel and are not associated with any clinical symptoms.
Plaque Rupture
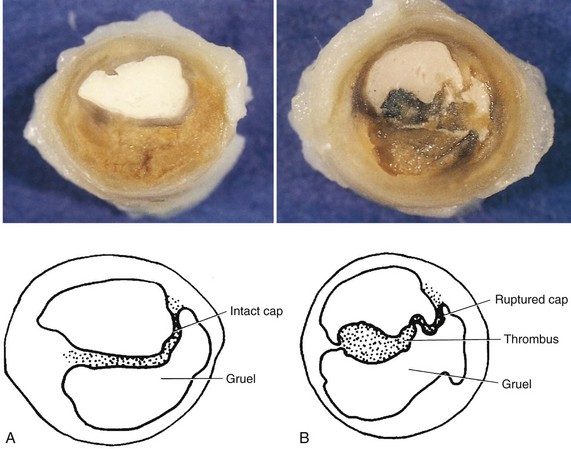
Atherosclerotic plaques differ with regard to their makeup, vulnerability to rupture, and tendency to form a blood clot. A “stable” or “nonvulnerable” atherosclerotic plaque has a relatively thick fibrous cap that separates it from contact with the blood and that covers a core containing a large amount of collagen and smooth muscle cells but a relatively small lipid pool (Figure 5-4). A stable plaque may produce significant luminal obstruction, but has a lower tendency to rupture or erode.2 Plaques that are prone to rupture are called “vulnerable” plaques because they have a thin cap of fibrous tissue over a large, soft, fatty center that separates it from the opening of the blood vessel. If the fibrous cap erodes or ruptures, the contents of the plaque (i.e., collagen, smooth muscle cells, tissue factor, inflammatory cells, and lipid material) are exposed to flowing blood.
The rupture of a vulnerable plaque may occur after the following: extreme physical activity (especially in someone unaccustomed to regular exercise), severe emotional trauma, sexual activity, exposure to illicit drugs (e.g., cocaine, amphetamines), exposure to cold, or acute infection.3 Contributing factors to plaque rupture may include shear stress (i.e., the frictional force from blood flow), coronary spasm at the site of the plaque, internal plaque changes, and the effects of risk factors (see Chapter 1).
Plaque disruption (rupture) is most likely to occur at vessel bifurcations because of the speed of blood flow and turbulence created at these areas. Three vulnerable sites for plaque disruption within the coronary arteries have been identified and include the following4:
Thrombus Development
If the cap of a vulnerable plaque erodes or ruptures, platelets stick to the damaged lining of the vessel and to each other within seconds and form a plug (Figure 5-5). “Sticky platelets” secrete several chemicals, including thromboxane A2. These substances stimulate vasoconstriction, reducing blood flow at the site. Aspirin, which is an antiplatelet agent, blocks the production of thromboxane A2, slowing down the clumping (i.e., aggregation) of platelets and lowering the risk of complete blockage of the vessel.
Other Causes of Acute Coronary Syndromes
Although a thrombus is the most common cause of blockage of a coronary artery, less commonly, an acute MI may occur as a result of coronary spasm (e.g., with cocaine abuse), abnormalities of coronary vessels, hypercoagulation, trauma to the coronary arteries, or coronary artery emboli (rare).5
Cocaine causes myocardial ischemia or MI by (1) increasing myocardial oxygen demand by increasing heart rate, blood pressure, and contractility; (2) decreasing oxygen supply via vasoconstriction; (3) inducing a prothrombotic state by stimulating platelet activation and altering the balance between procoagulant and anticoagulant factors; and (4) accelerating atherosclerosis.6 Although one study showed that two thirds of MI events occurred within 3 hours of cocaine ingestion,7 patients may not seek medical attention for hours to days after use.
The patient experiencing a cocaine-associated ACS may deny drug use and have atypical chest discomfort. Common cardiopulmonary complaints among cocaine users appear in Box 5-1.
Although there are no clear predictors for patients at risk of cocaine-associated ACS, the Cocaine-Associated Myocardial Infarction study retrospectively identified 130 patients who sustained a total of 136 cocaine-associated MI events. In this group, the majority of patients were young (mean age 38 years), nonwhite (72%), smokers (91%), and had a history of cocaine use in the preceding 24 hours (88%).6,8 A 2003 study showed that patients with cocaine-associated chest pain and positive cardiac biomarkers for MI had significant angiographic stenosis and of patients without positive serum markers, 18% still had significant disease by angiogram.9 Cardiac biomarkers are discussed later in this chapter.
Prinzmetal’s angina which is also called Prinzmetal’s variant angina or variant angina, is the result of intense spasm of a segment of a coronary artery. This variant angina may occur in otherwise healthy individuals (usually in their 40s or 50s) with no demonstrable coronary heart disease or in patients with a nonobstructive atheromatous plaque. In some studies, coronary arteriography in patients with Prinzmetal’s angina showed one-vessel CAD in 39% of patients and multivessel disease in 19%.10
Although the episode of coronary artery spasm can be precipitated by exercise, emotional stress, hyperventilation, or exposure to cold, it usually occurs at rest, often occurs between midnight and 8 AM, and may awaken the patient from sleep.11 Episodes may occur in clusters of two or three within 30 to 60 minutes. Although episodes usually last only a few minutes, this may be long enough to produce serious dysrhythmias including atrioventricular (AV) block and ventricular tachycardia (VT), as well as sudden death. If the spasm is prolonged, infarction may result.
Forms of Acute Coronary Syndromes
[Objective 2]
ACSs include unstable angina, NSTEMI, and STEMI.
Unstable Angina
The term angina refers to squeezing or tightening, rather than pain. The discomfort associated with angina occurs because of the stimulation of nerve endings by lactic acid and carbon dioxide that builds up in ischemic tissue. Common words used by patients experiencing angina to describe the sensation they are feeling are shown in Box 5-2. Some patients have difficulty describing their discomfort.
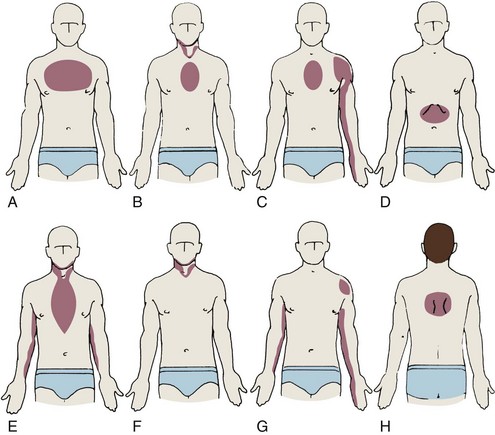
Chest discomfort associated with myocardial ischemia usually begins in the central or left chest and then radiates to the arm (especially the little finger [ulnar] side of the left arm), wrist, jaw, epigastrium, left shoulder, or between the shoulder blades (Figure 5-6). Ischemic chest discomfort is usually not sharp; it is not worsened by deep inspiration; it is not affected by moving muscles in the area where the discomfort is localized, nor is it positional in nature.
Stable (classic) angina remains relatively constant and predictable in terms of severity, signs and symptoms, precipitating events, and response to treatment. It is characterized by brief episodes of chest discomfort related to activities that increase the heart’s need for oxygen such as emotional upset, exercise or exertion, and exposure to cold weather. Possible related signs and symptoms are shown in Box 5-3. Symptoms typically last 2 to 5 minutes and occasionally 5 to 15 minutes. Prolonged discomfort (i.e., longer than 30 minutes) is uncommon in stable angina.
Unstable angina is characterized by one or more of the following:
Myocardial Infarction
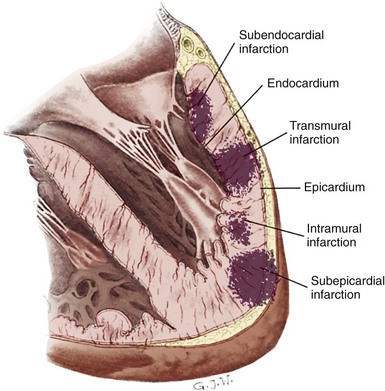
An MI occurs when blood flow to the heart muscle stops or is suddenly decreased long enough to cause cell death. The walls of the ventricles consist of an outer layer (the epicardium), middle layer (the myocardium), and an inner layer (the endocardium). The myocardium is subdivided into two areas. The innermost half of the myocardium is called the subendocardial area and the outermost half is called the subepicardial area. The main coronary arteries lie on the epicardial surface of the heart and feed this area first before supplying the heart’s inner layers with oxygenated blood. The endocardial and subendocardial areas of the myocardial wall are the least perfused areas of the heart and the most vulnerable to ischemia because these areas have a high demand for oxygen and are fed by the most distal branches of the coronary arteries. Transmural is a term used to describe ischemia, injury, or infarction that extends from the endocardium to the epicardium. For example, an infarction involving the entire thickness of the left ventricular wall is called a transmural MI. Possible locations of infarctions in the ventricular wall are shown in Figure 5-7.
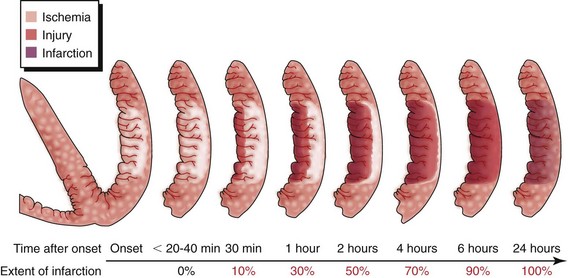
In the strictest sense, the term myocardial infarction relates to dead heart muscle tissue. In a practical sense, the term myocardial infarction is applied to the process that results in the death of myocardial tissue. Think of the “process” of MI as a continuum rather than the presence of dead heart tissue (Figure 5-8). Infarcted cells cannot respond to an electrical stimulus or provide any mechanical function. If efforts are made to recognize the process of MI, patients may be identified earlier. If they are promptly treated, the loss of heart tissue may be avoided.12
Universal Definition of Myocardial Infarction
In 1999, the European Society of Cardiology (ESC) and the American College of Cardiology (ACC) convened a conference to revise jointly the definition of MI. The definition for MI was examined from seven points of view: pathological, biochemical, electrocardiographic, imaging, clinical trials, epidemiological, and public policy. The consensus committee findings were published in 2000 in the European Heart Journal and Journal of the American College of Cardiology. The ESC, ACC, and the American Heart Association (AHA) convened, together with the World Heart Federation (WHF), a Global Task Force to update the 2000 document and an updated expert consensus document was published in 2007.13 Classifications of MI are shown in Table 5-1 and the criteria for acute MI appear in Box 5-4.
TABLE 5-1 Myocardial Infarction—Classifications
| Anatomic Classification14 | Description | |
|---|---|---|
| Transmural | Ischemic necrosis of the full thickness of the affected muscle segment(s), extending from the endocardium through the myocardium to the epicardium | |
| Nontransmural | Area of ischemic necrosis is limited to the endocardium or endocardium and myocardium, it does not extend through the full thickness of myocardial wall segment(s) | |
| Classification by Size13 | Description | |
| Microscopic | Focal necrosis | |
| Small | Less than 10% of the left ventricular (LV) myocardium | |
| Moderate | 10% to 30% of the LV myocardium | |
| Large | More than 30% of the LV myocardium | |
| Pathological Classification13 | Time Frame | Description |
| Evolving | Less than 6 hours | Minimal or no polymorphonuclear leukocytes may be seen |
| Acute | 6 hours to 7 days | Presence of polymorphonuclear leukocytes |
| Healing | 7 to 28 days | Presence of mononuclear cells and fibroblasts, absence of polymorphonuclear leukocytes |
| Healed | 29 days or more | Scar tissue without cellular infiltration |
| Classification by Location | ||
| Anterior | Inferior | Septal |
| Lateral | Inferobasal (posterior) | Right ventricular |
| Clinical Classification13 | Description | |
| Type 1 | Spontaneous myocardial infarction (MI) related to ischemia due to a primary coronary event such as plaque erosion and/or rupture, fissuring, or dissection | |
| Type 2 | MI secondary to ischemia due to either increased oxygen demand or decreased supply such as coronary artery spasm, coronary embolism, anemia, arrhythmias, hypertension, or hypotension | |
| Type 3 | Sudden unexpected cardiac death, including cardiac arrest, often with symptoms suggestive of myocardial ischemia, accompanied by presumably new ST elevation, or new left bundle branch block, or evidence of fresh thrombus in a coronary artery by angiography and/or at autopsy, but death occurring before blood samples could be obtained, or at a time before the appearance of cardiac biomarkers in the blood | |
| Type 4a | MI associated with percutaneous coronary intervention | |
| Type 4b | MI associated with stent thrombosis as documented by angiography or at autopsy | |
| Type 5 | MI associated with coronary artery bypass graft | |
Data from Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction: Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173–2195.13 Data from Bolooki HM, Bajzer CT: Acute myocardial infarction. In Cleveland Clinic: current clinical medicine, Philadelphia, 2009, Elsevier.14
Box 5-4 Criteria for Acute Myocardial Infarction13
History and Clinical Presentation12
[Objective 3]
Patient History
The average patient experiencing an ACS does not seek medical attention for 2 hours or more after the onset of ischemic chest pain symptoms.10,15 Women often delay longer than men do when seeking medical help. Common reasons that individuals delay in seeking medical care for ischemic-type chest discomfort are shown in Box 5-5.
Box 5-5 Common Reasons for Delays in Seeking Medical Care for Ischemic-type Chest Discomfort10,15
Not all chest discomfort is cardiac-related. Obtaining an accurate history is important to help determine if a patient’s signs and symptoms are most likely related to ischemia as a result of CAD. Because time is muscle when caring for patients with an ACS, it is important to ask targeted questions to determine the patient’s probability of an ACS and to not delay reperfusion therapy, if indicated. Important information to obtain when eliciting a targeted history is shown in Table 5-2.
TABLE 5-2 Acute Coronary Syndromes—Targeted History
Predisposing Factors
Studies have shown that the peak incidence of acute cardiac events is between 6 AM and noon.16–19 The early morning hours are associated with increases in blood pressure, heart rate, sympathetic nervous system activity, cortisol, and platelet aggregability. Some studies have shown that an MI is more likely to occur on Monday (as the patient transitions from weekend to work week) and during the winter months.16,20–22
A predisposing factor (i.e., trigger) is present in about 50% of patients experiencing an acute cardiac event.23 Examples include moderate to heavy physical exertion, unusual emotional stress, lack of sleep, overeating or use of alcohol, acute respiratory infection, or pulmonary embolism.19,24–27 Cocaine use may be a factor, particularly in patients younger than 40 years of age.
Typical Symptoms
[Objective 4]
Chest discomfort is the most common symptom of infarction. It is present in 75% to 80% of patients with acute MI. Patients experiencing a heart attack may describe the sensation they are feeling as similar to angina, or use words such as “heartburn,” “indigestion,” “dull,” “squeezing,” “gnawing,” “aching,” “tightness,” or “pressure.” The patient may describe his or her discomfort with a clenched fist held against the sternum (Levine’s sign). The discomfort typically lasts longer than 30 minutes. It may be constant or come and go, and occasionally may be relieved with belching.15
The ACC/AHA guidelines list the following as pain descriptions that are not characteristic of myocardial ischemia10:
Atypical Presentation
[Objective 6]
Not all patients experiencing an ACS present similarly. Atypical presentation refers to uncharacteristic signs and symptoms that are experienced by some patients. Atypical chest discomfort is localized to the chest area but may have musculoskeletal, positional, or pleuritic features. Examples of atypical presentations of STEMI are listed in Box 5-6.
Box 5-6 Atypical ST-Elevation Myocardial Infarction Signs and Symptoms
Atypical presentations of STEMI include the following28:
Patients experiencing an ACS who are most likely to present atypically include older adults, diabetic individuals, women, patients with prior cardiac surgery, and patients during the immediate postoperative period after noncardiac surgery.5
ACLS Pearl
Researchers compared African-American, Hispanic, and Caucasian women’s prodromal and acute symptoms of MI.29 Symptom severity and frequency were compared among racial groups. Among the women, 96% reported prodromal symptoms. Unusual fatigue (73%) and sleep disturbance (50%) were the most frequent. Eighteen symptoms differed significantly by race. African-American women reported higher frequencies of 10 symptoms than did Hispanic or Caucasian women. Thirty-six percent reported prodromal chest discomfort. Hispanic women reported more pain/discomfort symptoms than did African-American or Caucasian women. Minority women reported more acute symptoms. The most frequent symptom, regardless of race, was shortness of breath (63%); 22 symptoms differed by race. In total, 28% of Hispanic, 38% of African-American, and 42% of Caucasian women reported no chest pain/discomfort. These researchers concluded that prodromal and acute symptoms of MI differed significantly according to race.
Physical Examination
[Objective 3]
Although the physical examination for patients being evaluated for possible ACS is often normal, performing a physical examination is important to identify potential precipitating causes of myocardial ischemia (e.g., such as uncontrolled hypertension, gastrointestinal [GI] bleeding), assess the hemodynamic impact of the ischemic event, and identify coexisting conditions (e.g., pulmonary disease, malignancies) that could influence treatment decisions.10 Because the goals of reperfusion therapy are to give fibrinolytics within 30 minutes of patient arrival or provide percutaneous coronary intervention (PCI) within 90 minutes of arrival, the targeted history and focused physical examination must be performed quickly and efficiently. The physical examination should include the following:
Patient Evaluation
Electrocardiogram Findings12
[Objective 7]
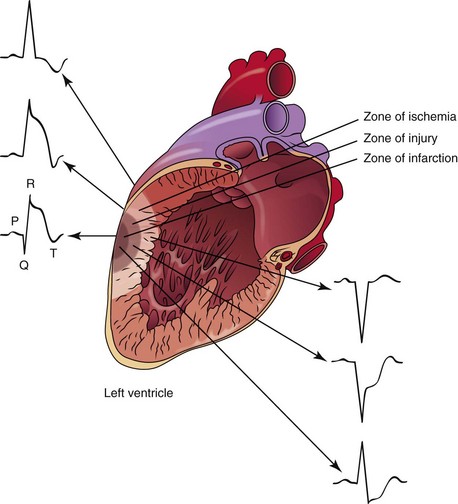
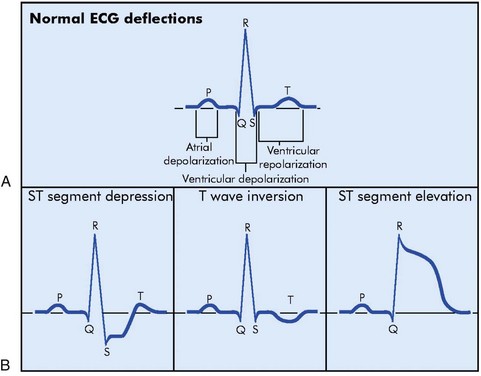
The sudden blockage of a coronary artery may result in ischemia, injury, or death of the area of the myocardium supplied by the affected artery. The area supplied by the blocked artery goes through a sequence of events that has been identified as “zones” of ischemia, injury, and infarction. Each zone is associated with characteristic ECG changes (Figure 5-9).
Myocardial Ischemia12
Myocardial Injury12
[Objective 7]
ECG evidence of myocardial injury in progress can be seen on the ECG as ST elevation in the leads facing the affected area (see Figure 5-10). In leads opposite the affected area, ST depression (i.e., reciprocal changes) may be seen.
For men 40 years of age and older, the threshold value for abnormal J-point elevation is 2 mm in leads V2 and V3 and 1 mm in all other leads. For men younger than 40 years of age, the threshold value for abnormal J-point elevation in leads V2 and V3 is 2.5 mm. For women, the threshold value for abnormal J-point elevation is 1.5 mm in leads V2 and V3 and greater than 1 mm in all other leads. For men and women, the threshold for abnormal J-point elevation in V3R and V4R is 0.5 mm, except for males younger than 30 years of age, for whom 1 mm is more appropriate. For men and women, the threshold value for abnormal J-point elevation in leads V7 through V9 is 0.5 mm.30–32
Myocardial Infarction12
[Objective 7]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree