The aim of the present study was to assess the feasibility, safety, and long-term results of remote magnetic navigation in arrhythmias associated with complex congenital heart disease (CHD). The improved outcomes for CHD resulted in an increased number of complex arrhythmias requiring distinctive ablation techniques. Thirty-six patients with CHD (age 35 ± 19 years, 21 male) were divided into 3 complexity groups and underwent 43 radiofrequency catheter ablation procedures using the magnetic navigation system (including 7 redo ablations) in combination with the CARTO RMT system. A total of 59 tachyarrhythmias were identified. Most patients had surgical scar-related tachycardia (25 focal, including 4 microreentrant atrial tachycardia, and 27 macroreentrant atrial tachycardia). Four accessory pathways and three ventricular tachycardias were diagnosed and treated. In 31 patients, ablation was successful, with an end point of noninducibility (86%). The success rate for CHD complexity of type I, II, and III was 50%, 88%, and 89%, respectively. The mean procedure and fluoroscopy time was 216 ± 101 minutes and 40 ± 34 minutes, respectively. The number of radiofrequency applications was 42 ± 47. No major complications related to the procedures occurred. Of the patients, 67% remained free of recurrence during a mean follow-up of 26 ± 4 months. Recurrence developed in 0%, 16%, and 45% of patients with CHD type I, II, and III, respectively. In conclusion, the magnetic navigation system is feasible to treat arrhythmias with reasonable success rates and good long-term outcomes in adult patients with CHD. The use of the magnetic navigation system offers advantages in complex anatomic situations.
Catheter ablation became first-line therapy for several forms of arrhythmias in the 1990s, with reports of successful ablations in patients with congenital heart disease (CHD). However, catheter ablation of patients with CHD still presents a challenge for the electrophysiologist. The cardiac anatomy is usually very different from that of the normal population (often children with small hearts, anatomic abnormalities, surgical reconstructions), making navigation difficult and resulting in a greater risk of complications. The differential diagnosis of the relevant arrhythmia is often more challenging in patients with CHD, a crucial point in the planning of an adequate and successful ablation strategy. The magnetic navigation system (MNS) has advantages that could help to overcome these mentioned difficulties, including improved safety owing to the atraumatic catheter design, less restricted and reproducible catheter movement, and improved catheter stability. Despite these advantages of the MNS, the long-term results in adults with CHD are sparse. Our purpose was to evaluate the acute and long-term safety and efficacy of the MNS for the ablation of arrhythmias in patients with CHD.
Methods
A total of 36 consecutive patients with CHD (age 35 ± 19 years, 21 male) with symptomatic, drug-refractory tachyarrhythmias underwent ablation from March 2007 to April 2011 and were included in the present study. Incessant drug-refractory arrhythmias were seen in 31% of the patients. The patients were divided into 3 categories of complexity according to the American College of Cardiology/American Heart Association 2008 guidelines: grade I for simple CHD, grade II for CHD of moderate complexity; and grade III for CHD of great complexity. Of the 36 patients, 29 had undergone previous heart surgery. All patients provided written informed consent for the ablation procedure. A 12-lead electrocardiogram at rest, laboratory tests, radiographic thorax image, and 2-dimensional echocardiogram were acquired from all patients within the month before and 48 hours after the procedure. A transesophageal echocardiogram was performed, if considered necessary. The procedures were performed during a fasting state, using local or general anesthesia. In general, patients were instructed to stop taking antiarrhythmic drugs for a period of at least 4 half lives before undergoing the procedure. Exceptions were made for amiodarone use and for patients with ongoing tachycardias even with antiarrhythmic drugs or requiring urgent catheter ablation procedures.
The ablation procedures were executed by the same group of our senior electrophysiologist and were performed in all patients using MNS (Stereotaxis Niobe II, Stereotaxis, St. Louis, Missouri) implemented in an electrophysiology laboratory equipped with a Siemens Axiom Artis (Siemens, Erlangen, Germany) fluoroscopy system. The principles and use of the MNS have been previously reported. The Niobe II MNS consists of 2 permanent magnets situated on both sides of the patient. The system uses a computer-controlled workstation (Navigant, Sterotaxis, St. Louis, Missouri) to allow changes in the magnetic field orientation to navigate the ablation catheter. A combined field strength of 0.08 or 0.1 T was used. The following ablation catheters were used: Celsius RMT (4 mm; Biosense Webster, Diamond Bar, California) and NaviStar RMT ThermoCool and Navistar RMT DS (8 mm). Electroanatomic mapping was performed in all patients using the CARTO RMT (Biosense Webster, Diamond Bar, California) system.
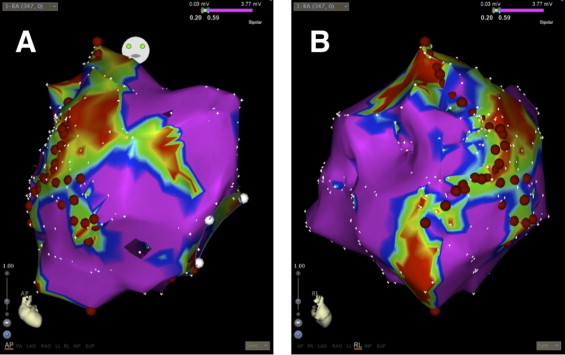
Atrial tachycardias (ATs) were classified as centrifugal AT (CAT), including a focal and microreentrant mechanism or macroreentrant atrial tachycardia (MRAT). These classifications were made using atrial activation maps. A microreentrant mechanism was diagnosed according to the entrainability combined with a centrifugal activation pattern. For both centrifugal and macroreentrant atrial tachycardia, the end points of procedural success were defined as termination of the tachycardia and noninducibility. For circus movement tachycardia, it was the elimination of accessory pathway conduction, and for ventricular tachycardia, it was noninducibility. If multiple sustained arrhythmias could be induced in the same patient, each was ablated consecutively. The ablation strategies were tailored to the type of arrhythmia. For CAT, the radiofrequency applications were applied at the site of the earliest activation. In the case of MRAT, linear ablation was performed to interrupt the reentrant circuit ( Figure 1 ).
The following parameters were analyzed: acute success rate, number of baffle or transseptal punctures, fluoroscopy time, procedure time, number of radiofrequency applications, total radiofrequency application duration, recurrence during follow-up, and complications. The acute success rate was assessed according to the terms reported. The fluoroscopy and procedure times (beginning at subcutaneous injection of lidocaine to the groin and ending when the catheters were removed) were recorded in the clinical procedure log and included a 30-minute waiting time. Any adverse event during the procedure, before hospital discharge, or reported by the general physician during follow-up was investigated by a trained electrophysiologist and was considered as a complication if the event could be related to the procedure. Complications were categorized as major (pericardial effusion or/and tamponade, permanent atrioventricular block, stroke, major bleeding or death) or minor (minor bleeding, transient ischemic attack and temporary atrioventricular block).
Follow-up visits were scheduled for all patients at the outpatient clinic of the cardiology department (Erasmus Medical Center) 3 months after the procedure and every 3 months thereafter. After 1 year, the patients were followed up by their referring congenital or pediatric cardiologist.
The parameters obtained from the registry were analyzed using SPSS, version 15.0 (SPSS, Chicago, Illinois). The patient demographic and baseline characteristics were presented as the mean ± SD. Cumulative Kaplan-Meier survival curves were constructed for recurrence-free survival, and a log-rank test was performed.
Results
The baseline patient characteristics are listed in Table 1 . The underlying disease of all 36 patients is listed in Table 2 . Of the 36 patients, 29 had undergone previous heart surgery ( Table 3 ). A total of 59 arrhythmias were identified during the 43 procedures. In the 29 patients who had undergone surgical repair, 90% of the arrhythmias proved to be AT (45% CAT and 55% MRAT). Nine patients had 13 CATs, and 21 MRATs were seen in 17 patients. Three patients had both, with a total of 10 arrhythmias. Of the 17 patients with MRAT, 4 had 2 reentry circuits. Five patients had typical atrial flutter; the rest of the 19 MRATs were atypical atrial flutter mechanisms related to the surgical incisions. In the patient group with a history of surgical repair, 2 patients had circus movement tachycardia (1 left- and 1 right-sided accessory pathway) and 3 patients had ventricular tachycardia. In these postoperative patients, 45 arrhythmias could be ablated successfully (92%). One CAT, 2 MRATs, and 1 circus movement tachycardia using a left-sided accessory pathway could not be terminated by ablation, and electrical cardioversion was performed, resulting in a sinus rhythm. For 1 MRAT, the most critical part of the circuit could not be reached endocardially and was therefore not successful.
| Characteristic | Value |
|---|---|
| Age (years) | |
| Mean ± SD | 35 ± 19 |
| Range | 2–77 |
| Gender | |
| Male | 21 |
| Female | 15 |
| Congenital heart disease complexity | |
| Grade I | 4 (11%) |
| Grade II | 15 (42%) |
| Grade III | 17 (47%) |
| Amiodarone prescription | 8 (22%) |
| Sotalol prescription | 14 (39%) |
| β Blocker prescription | 8 (22%) |
| Digoxin prescription | 1 (3%) |
| Verapamil prescription | 3 (8%) |
| Heart surgery (number of operations) | 1.7 ± 0.8 |
| Main diagnosis (n = 36) | Patients (n) | Age at Ablation (years) |
|---|---|---|
| Aortic valve stenosis | 1 (3%) | 31 ± 0 |
| Atrial septal defect | 4 (11%) | 56 ± 9 |
| Sinus venosus defect | 2 (6%) | 54 ± 18 |
| Atrioventricular septal defect | 2 (6%) | 14 ± 1 |
| Double outlet right ventricle | 1 (3%) | 42 ± 0 |
| Ebstein anomaly | 6 (17%) | 31 ± 18 |
| Patent ductus arteriosus | 1 (3%) | 77 ± 0 |
| Pulmonary valve stenosis | 2 (6%) | 28 ± 6 |
| Transposition of the great arteries | 10 (28%) | 25 ± 13 |
| Tetralogy of Fallot | 2 (6%) | 59 ± 6 |
| Tricuspid artresia | 4 (11%) | 24 ± 5 |
| Ventricular septal defect | 1 (3%) | 42 ± 0 |
| Main Operation (n = 29) | Patients (n) | Age at Surgery (years) | Age at Ablation (years) |
|---|---|---|---|
| Atrial septal defect closure | 3 (10%) | 40 ± 27 | 55 ± 9 |
| Atrioventricular septal defect closure | 2 (7%) | 3 ± 2 | 14 ± 1 |
| Fontan procedure | 7 (24%) | 4 ± 3 | 23 ± 8 |
| Mustard-Senning procedure | 8 (28%) | 7 ± 10 | 29 ± 14 |
| Pulmonary valvulotomy | 2 (7%) | 1 ± 0 | 28 ± 7 |
| Tetralogy of Fallot correction | 3 (10%) | 12 ± 7 | 53 ± 11 |
| Tricuspid valve surgery | 3 (10%) | 6 ± 1 | 42 ± 8 |
| Ventricular septal defect closure | 1 (3%) | 7 ± 0 | 42 ± 0 |
In the 7 patients with CHD without previous heart surgery 10 arrhythmias were identified. Eighty percent of these arrhythmias were AT (63% CAT and 38% MRAT). Three CATs were diagnosed in 3 patients, and 2 MRATs were seen in 2 patients. One patient had both CAT and MRAT, and 3 arrhythmias were identified. In 2 patients, circus movement tachycardia with right-sided accessory pathways was identified. In the 7 patients with CHD and without surgery, 5 of the arrhythmias could be ablated (50%). However, 2 CATs and 3 MRATs could not be terminated, and electrical cardioversion was performed. Two figure-of-8 MRATs could not be ablated owing to the presence of a percutaneous atrial septal defect closure device.
Of those with complexity score I, 25% had undergone previous surgery. A total of 6 arrhythmias were identified, including 3 CATs and 2 MRATs. Two CATs and one MRAT could be ablated successfully, for an overall success rate of 50%.
Of those with complexity score II, 73% of these patients had undergone earlier surgery. A total of 24 tachycardias were targeted, including 10 CATs and 10 MRATs. The acute success rate for CAT was 100% and 80% for MRAT.
Of those with complexity score III, all the patients had a history of surgery. There were 29 inducible tachycardias: 12 CATs and 15 MRATs. Of the CATs and MRATs, 92% and 87% reached the end point of noninducibilty after the ablation procedure, respectively.
Ablation was successful in 31 patients (86%), with an end point of noninducibility. The success rate was 88% (22 of 25) for CAT, 81% (22 of 27) for MRAT, 100% (3 of 3) for circus movement tachycardia using a right-sided accessory pathway, and 100% (3 of 3) for ventricular tachycardia. The circus movement tachycardia with a left-sided accessory pathway could not be terminated by radiofrequency ablation. The overall acute success rate for patients with CHD complexity type I, II, and III was 50%, 88%, and 89%, respectively.
Biatrial mapping was necessary in 15 of the 43 procedures, with a retrograde approach in most cases. A transseptal puncture was needed in 3 patients, and 1 baffle puncture was performed. Crossover to manual cryoablation was necessary in 3 procedures for successful ablation (septal CAT, right posterior accessory pathway, and typical flutter). In 1 patient, crossover to manual cryoablation was tried but did not result in successful ablation. The mean fluoroscopy and procedure time was 40 ± 34 minutes and 216 ± 101 minutes, respectively. The mean number of radiofrequency applications was 42 ± 47, and the mean radiofrequency application duration was 1,217 ± 1,035 seconds. No major complications were related to the procedures. Three minor complications were all groin hematomas.
Redo procedures were performed in 19% of all patients. Of these, 57% were in CHD grade III, 43% in grade II, and 0% in grade I. In 3 of these patients, the initial targeted arrhythmia was induced (1 CAT, 1 MRAT, and 1 typical flutter), and in 4 patients, a different arrhythmia was identified.
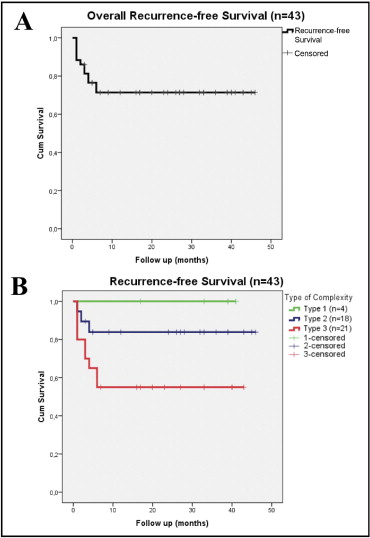
Of the 36 patients, 22 (61%) remained free of recurrence during a mean follow-up of 26 ± 14 months ( Figure 2 ). All patients with recurrence presented within the first 6 months after procedure. After redo procedures, 24 patients (67%) did not experience any recurrence. During follow-up, recurrence occurred mostly in patients with type III CHD complexity (45%): transposition of the great arteries (56%), tricuspid valve atresia (33%), and double outlet of the right ventricle (11%). In type II complexity, 16% had recurrence (p = 0.075): Ebstein anomaly (67%) and sinus venosus defect (33%). After successful ablation, none of the patients with type CHD I experienced a recurrence during the follow-up period ( Figure 3 ).