Controls
ASD
P-value
WBC (109/L)
6.91 ± 0.42
7.96 ± 0.37
0.070
Neu [109/L (%)]
3.38 ± 0.34 (47.4 ± 1.8 %)
3.91 ± 0.36 (48.1 ± 3.2 %)
0.117
Lym [109/L (%)]
2.75 ± 0.12 (41.2 ± 1.8 %)
3.07 ± 0.26 (39.2 ± 3.2 %)
0.283
Mon [109/L (%)]
0.49 ± 0.03 (7.2 ± 0.4 %)
0.67 ± 0.04 (8.5 ± 0.6 %)
0.003
Eos [109/L (%)]
0.27 ± 0.04 (3.9 ± 0.4 %)
0.29 ± 0.04 (3.8 ± 0.5 %)
0.453
Bas [109/L (%)]
0.03 ± 0.01 (0.4 ± 0.04 %)
0.03 ± 0.003 (0.4 ± 0.04 %)
0.592
RBC (1012/L)
5.04 ± 0.08
5.21 ± 0.20
0.777
HGB (g/L)
143.6 ± 2.5
143.4 ± 5.6
0.350
HCT (%)
43.0 ± 0.7
43.6 ± 1.8
0.484
MCV (fL)
85.5 ± 1.0
83.7 ± 1.4
0.315
MCH (pg)
28.5 ± 0.3
27.6 ± 0.4
0.091
MCHC (g/L)
333.6 ± 1.6
329.5 ± 2.2
0.153
RDW-CV (%)
12.0 ± 0.1
12.3 ± 0.1
0.153
RDW-SD (fL)
41.5 ± 0.6
41.2 ± 0.7
0.702
PLT (109/L)
290.1 ± 12.6
281.9 ± 17.4
0.708
MPV (fL)
8.8 ± 0.2
8.5 ± 0.3
0.504
PDW
16.3 ± 0.1
16.3 ± 0.1
0.828
PCT (%)
0.26 ± 0.01
0.24 ± 0.01
0.330
3.2 Cytokine Profile and Oxidative Stress Assay
The plasma level of IL-8 was significantly higher in children with ASD compared to controls (p = 0.003, Fig. 1). There were no significant differences in the other proinflammatory markers IL-1ß and TNF-α or in TBARS between the ASD and control groups.
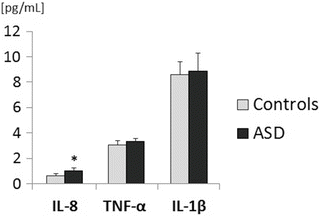

Fig. 1
Cytokine profile in autism spectrum disorders (ASD) and controls. IL-8 interleukin 8, TNF-α tumor necrosis factor alpha, IL-1β interleukin 1ß. Data are means ± SE, *p < 0.01
4 Discussion
Despite the fact that the etiology and pathogenesis of ASD are still unclear, it is suggested that there may be an association with immune dysfunction (Onore et al. 2012; Ashwood et al. 2011a). Cytokines are proteins that control the intensity, duration, and type of immune response. However, cytokines are also involved in brain development and synaptic functions including processes of differentiation, migration, proliferation, and behavioral impairments (Onore et al. 2012; Ashwood et al. 2011a). For example, neuropoietic cytokines, such as IL-6, can directly alter cortical neuron dendrite development, neural activity, long-term potentiation, and neurodevelopment, which all may influence behavior (Ashwood et al. 2011a, b; Gilmore et al. 2005). Similarly, IL-1β nad TNF-α have been linked with neurite growth and the regulation of synaptic plasticity in the hippocampus (Cacci et al. 2008). Therefore, the abnormalities in the cytokine profile could represent an important potential pathomechanism leading to impaired neuronal development in ASD (Ashwood et al. 2011a).
In the present study, we found a higher number of monocytes and increased plasma concentrations of IL-8, which indicates abnormal inflammatory activity in children with ASD. These results are in agreement with other findings which have reported increases of a number of cytokines, including IL-8, in plasma of these children (Ashwood et al. 2011a). Notably, the finding of increased plasma IL-8, a chemoattractant cytokine of important role in the inflammatory process, corresponds to IL-8 increases seen in the brain and cerebrospinal fluid in ASD (Vargas et al. 2005). Higher plasma levels of other chemokines, such as MCP-1 or eotaxin, have also been found in ASD (Ashwood et al. 2011b), although in the present study we did not observe increases in IL-1β or TNF-α, Thus, it seems that IL-8 is the most sensitive pro-inflammatory factor in ASD children. However, the reason why this chemokine is selectively increased in ASD is currently unclear. Generally, chemokines produced by macrophages and other cell types, such as epithelial and endothelial cells, have chemotactic activity toward neutrophils and play important roles in the innate immune response. It is assumed that the elevation of IL-8 in the peripheral circulation indicates the activation of innate immunity. However, higher IL-8 plasma levels may also result from IL-17 secretion by Th17 cells activated in response to subclinical infections in epithelial or endothelial cells in ASD (Suzuki et al. 2011). Furthermore, IL-8 is associated with impaired stereotypical behavior, and negatively correlates with cognitive and adaptive ability, and with receptive and expressive language (Ashwood et al. 2011a). It seems that the question of whether the enhancement of IL-8 is a result of abnormal immune response in children with ASD or is only a reflection of the chronic autism-related symptoms remains unresolved. In this context, it is currently questionable whether the plasma increase of IL-8 has a pathogenetic role in autism, and whether anti-IL-8 therapy could be useful.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


