Acquired Immunodeficiency Syndrome
Respiratory infections are a major cause of morbidity and mortality in patients with acquired immunodeficiency syndrome (AIDS) (1). It is estimated that >70% of patients with AIDS will suffer at least one pulmonary infection during the course of their illness (2). The type of pulmonary infection occurring in an human immunodeficiency virus (HIV)-infected patient depends on the stage of the HIV infection, history of prior infection, virulence of the infecting organism, and other host-related factors, such as the disease exposure and geographic location (3,4,5).
Immunosuppression associated with HIV infection increases the susceptibility to a variety of pulmonary infections, which represent at least 65% of all AIDS-defining illnesses (see Table 7.1) (2). The risk of pulmonary infection is influenced by the patient’s immune status, being greatest at a CD4 lymphocyte count of <200 cells per mm3 (2,6,7). Opportunistic lung infections seldom occur in patients with >200 CD4 lymphocytes per mm3. HIV-positive patients with >500 CD4 lymphocytes per mm3 are only mildly immunocompromised and are at increased risk only for bronchial infections and bacterial pneumonia. However, patients with <200 CD4 lymphocytes per mm3 are predisposed to pulmonary infections caused by a variety of opportunistic and nonopportunistic pathogens, including Pneumocystis pneumonia (PCP), nontuberculous mycobacterial infection, and recurrent bacterial pneumonia. At CD4 lymphocyte counts <100 cell per mm3, and especially at CD4 lymphocyte counts <50 cell per mm3, the patients are prone to pulmonary infection by endemic fungi and cytomegalovirus (CMV) (7,8), and to develop disseminated infection by Mycobacterium avium-intracellulare complex (MAC).
The most common organisms causing pulmonary infection in HIV-positive patients are gram-positive and gram-negative bacteria, Pneumocystis jiroveci (previously known as P. carinii), Mycobacterium tuberculosis, and MAC (9,10,11). A resurgence of tuberculosis has been seen worldwide, largely related to the AIDS epidemic (12).
In recent years, there have been changing patterns in the epidemiology and treatment of HIV infection (9).
The introduction of highly active antiretroviral therapy (HAART) and the use of prophylactic antibiotics has been associated with a dramatic reduction in the number of HIV-positive patients presenting with respiratory infections (13). HAART has resulted in a decrease in the viral load, an increase in the mean CD4 lymphocyte count causing reduction of the morbidity and mortality from opportunistic infection, and a considerable increase in survival rates (13). However, pulmonary parenchymal complications remain the main cause of morbidity and mortality in these patients (14). Early diagnosis and treatment of these complications is important to improve survival.
The introduction of highly active antiretroviral therapy (HAART) and the use of prophylactic antibiotics has been associated with a dramatic reduction in the number of HIV-positive patients presenting with respiratory infections (13). HAART has resulted in a decrease in the viral load, an increase in the mean CD4 lymphocyte count causing reduction of the morbidity and mortality from opportunistic infection, and a considerable increase in survival rates (13). However, pulmonary parenchymal complications remain the main cause of morbidity and mortality in these patients (14). Early diagnosis and treatment of these complications is important to improve survival.
TABLE 7.1 Infections In Patients With Acquired Immunodeficiency Syndrome | ||
|---|---|---|
|
In most patients with AIDS, a confident diagnosis of the pulmonary complications can be made from a combination of clinical, radiographic, and laboratory findings (15). However, 5% to 10% of patients with AIDS and pulmonary disease have normal or questionable radiographic findings (6). High-resolution computed tomography (CT) scan is more sensitive than radiography in demonstrating parenchymal abnormalities in patients with AIDS and is superior to the radiograph in the differential diagnosis of the pulmonary complications seen in these patients (9,14,16).
TABLE 7.2 Bacterial Infections in Acquired Immunodeficiency Syndrome | |
|---|---|
|
TABLE 7.3 Radiographic Findings of Bacterial Infections in Acquired Immunodeficiency Syndrome | |
|---|---|
|
Bacteria
Bacterial pneumonia and pyogenic bronchitis are the commonest causes of pulmonary infection in patients with AIDS, being particularly frequent among intravenous drug users and smokers (17,18). Bacterial pneumonias are most commonly due to Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Streptococcus viridans, and Staphylococcus aureus (see Table 7.2) (1,2,17,19). In an autopsy-based study of 233 HIV-positive patients with pulmonary complications, bacterial pneumonia due to P. aeruginosa and S. aureus was the most frequent pulmonary complication (4,20). S. pneumoniae has been identified as the leading cause of community-acquired bacterial pneumonia in HIV-positive patients seen at all levels of CD4
lymphocyte count (21,22). The incidence of S. pneumoniae infection in HIV-positive individuals is 5 to 18 times greater than that in the general population, and the development of S. pneumoniae septicemia is 100 times greater (23).
lymphocyte count (21,22). The incidence of S. pneumoniae infection in HIV-positive individuals is 5 to 18 times greater than that in the general population, and the development of S. pneumoniae septicemia is 100 times greater (23).
Nosocomial pneumonias among HIV-positive patients are indistinguishable from those occurring in other hospitalized patients (24). As reported by Afessa et al. (4), P. aeruginosa is a common cause of both community-acquired and nosocomial bacterial pneumonia in hospitalized patients with HIV, especially in those with low leukocyte and CD4 lymphocyte counts.
The diagnosis of bacterial infection in patients with AIDS may be established by the combination of clinical, radiographic, and microbiologic findings (see Table 7.3). Respiratory symptoms are common and patients can present with dyspnea, cough, and, less commonly, pleuritic chest pain. A productive cough with purulent sputum suggests a bacterial infection, whereas a nonproductive cough and dyspnea are more characteristic of PCP or other fungal infections. Patients with pneumonia may be febrile, tachycardic, and tachypneic.
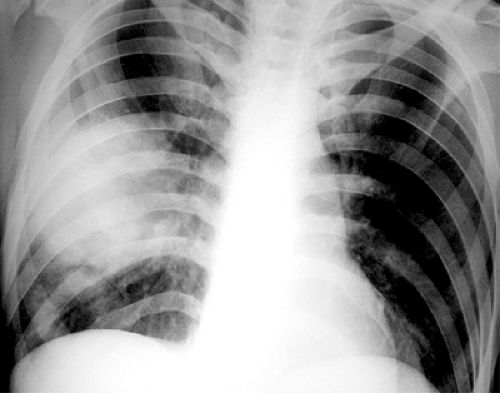
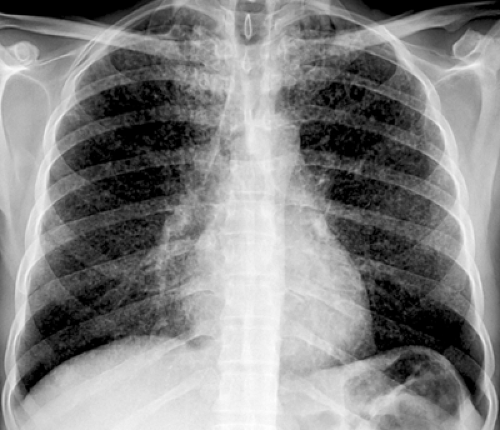
The most common chest radiographic features of pneumonia include single or multiple areas of focal consolidation, in either a patchy or lobar distribution (25). Lobar pneumonia is characterized by the spread of bacteria and inflammatory exudates between the alveolar airspaces and crossing segmental boundaries (nonsegmental consolidation), a pattern seen most commonly in S. pneumoniae pneumonia. A rounded appearance (rounded pneumonia) may also be seen, particularly in S. pneumoniae pneumonia (see Fig. 7.1) (22,25,26,27). Bronchopneumonia, characterized by patchy unilateral or bilateral consolidation, can result from a variety of gram-positive and gram-negative bacteria, most commonly Staphylococcus, Streptococcus, Pseudomonas, Klebsiella, Enterobacter, and Haemophilus species. HIV-positive patients who are intravenous drug users have an increased prevalence of cavitated P. aeruginosa
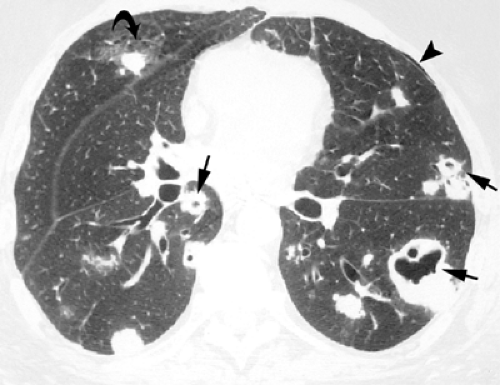
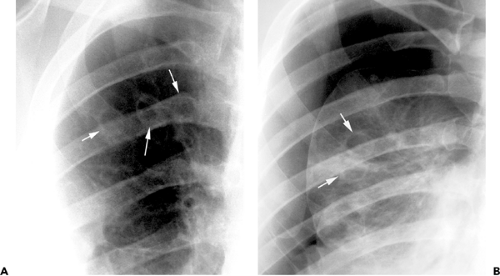
pneumonia, recurrent staphylococcal infections, and septic emboli (see Fig. 7.2). These various infections may be associated with complications such as pneumothorax and empyema (see Fig. 7.3) (25,28).
pneumonia, recurrent staphylococcal infections, and septic emboli (see Fig. 7.2). These various infections may be associated with complications such as pneumothorax and empyema (see Fig. 7.3) (25,28).
Although a focal consolidation is highly suggestive of bacterial pneumonia, differentiation from atypical patterns of opportunistic infections is often impossible on the basis of radiographic findings. Conversely, atypical patterns, including bilateral diffuse opacities, are not uncommon manifestations of bacterial pneumonia (14,27,29,30). Uncomplicated bacterial pneumonia may have a clinical and radiographic response to antibiotic therapy, which is similar to immunocompetent individuals undergoing treatment for community-acquired pneumonia (14,27,29,30).
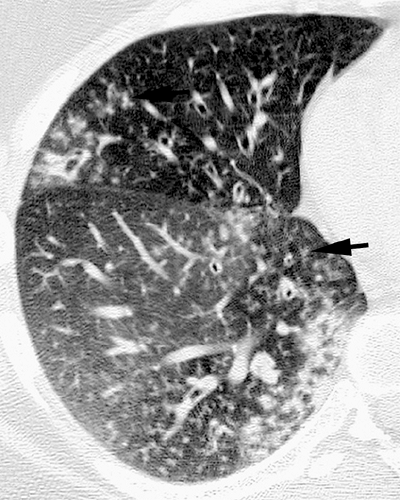
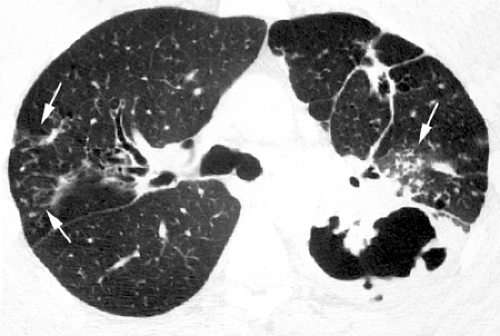
Pyogenic airway diseases, including infectious bronchitis and bronchiolitis, are seen with increasing frequency in HIV-positive patients (31,32). They are characterized histologically by inflammation of the bronchi and bronchioles and the presence of an inflammatory exudate and mucus in airway lumen (33,34). Chest radiographs are usually normal or may show subtle bronchial wall thickening, presenting as “tram tracks.” CT scan is of limited value in the assessment of bronchitis but is often helpful in the diagnosis of bronchiolitis and early bronchopneumonia. The characteristic high-resolution CT scan findings of infectious bronchiolitis and bronchopneumonia include a “tree-in-bud” pattern characterized by: (a) small centrilobular nodular opacities representing bronchioles impacted with inflammatory material and peribronchiolar inflammation being seen in cross-section, (b) branching linear opacities corresponding to abnormal bronchioles being seen along their long axis, and (c) focal areas of consolidation due to bronchopneumonia (see Fig. 7.4) (34).
Nosocomial pneumonias in AIDS are indistinguishable from those occurring in other hospitalized patients.
Mycobacteria
Mycobacterium tuberculosis
Mycobacterium tuberculosis remains an important respiratory pathogen in HIV-positive patients. After decades of decreasing incidence, tuberculosis has reemerged as an important infection worldwide (35). The main factor responsible for the significant increase of tuberculosis since the mid-1980s has been the increased prevalence of HIV infection. The incidence of tuberculosis in patients with AIDS is 200 to 500 times greater than that of the general population (36,37). HIV infection is the strongest known risk factor for progression from latent to active tuberculosis (38). Of the estimated 42 million individuals infected with HIV worldwide, >25% have active tuberculosis (38). Most of these patients live in countries with limited health care resources in Africa and Asia. The incidence of tuberculosis in these countries is increasing (24).
The manifestations of tuberculosis in patients with AIDS are influenced by the degree of immunosuppression (see Table 7.4) (5). The radiologic findings in patients with mild
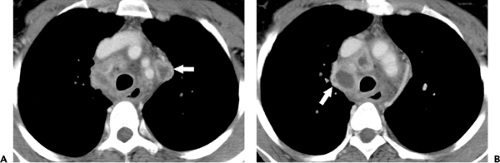
immunosuppression (>200 CD4 cells per mm3) are usually similar to those of postprimary disease in the healthy host (39,40,41). The abnormalities include focal areas of consolidation and nodular opacities involving mainly the apical and posterior segments of the upper lobes. Cavitation occurs in approximately 20% of patients and lymph node enlargement in 10%. In patients with severe immunosuppression (CD4 lymphocyte count <200 per mm3) the findings tend to resemble those of primary tuberculosis (36,41). The predominant abnormalities include hilar and/or mediastinal lymph node enlargement and airspace consolidation. Enlarged hilar and/or mediastinal nodes are evident on the radiograph in 30% to 60% of patients and on CT scan in 70% to 90% (36,37,42). The enlarged nodes usually have decreased attenuation on CT scan and often show rim enhancement following intravenous administration of contrast (see Fig. 7.5) (42,43). The decreased number of T lymphocytes and deficient delayed-type hypersensitivity reaction in patients with AIDS result in impaired ability to form granulomas, kill the bacilli, and localize the disease. Therefore these patients have increased prevalence of miliary tuberculosis (see Fig. 7.6) (36).
immunosuppression (>200 CD4 cells per mm3) are usually similar to those of postprimary disease in the healthy host (39,40,41). The abnormalities include focal areas of consolidation and nodular opacities involving mainly the apical and posterior segments of the upper lobes. Cavitation occurs in approximately 20% of patients and lymph node enlargement in 10%. In patients with severe immunosuppression (CD4 lymphocyte count <200 per mm3) the findings tend to resemble those of primary tuberculosis (36,41). The predominant abnormalities include hilar and/or mediastinal lymph node enlargement and airspace consolidation. Enlarged hilar and/or mediastinal nodes are evident on the radiograph in 30% to 60% of patients and on CT scan in 70% to 90% (36,37,42). The enlarged nodes usually have decreased attenuation on CT scan and often show rim enhancement following intravenous administration of contrast (see Fig. 7.5) (42,43). The decreased number of T lymphocytes and deficient delayed-type hypersensitivity reaction in patients with AIDS result in impaired ability to form granulomas, kill the bacilli, and localize the disease. Therefore these patients have increased prevalence of miliary tuberculosis (see Fig. 7.6) (36).
TABLE 7.4 Tuberculosis in Acquired Immunodeficiency Syndrome | ||
|---|---|---|
|
Although the chest radiograph plays an important role in the diagnosis of tuberculosis it may be normal in up to 15% of patients with AIDS and sputum culture-positive disease (44,45). Furthermore, the radiograph may fail to demonstrate characteristic findings of tuberculosis in patients with active disease. In a retrospective study of 133 patients with AIDS who had culture-positive tuberculosis, chest radiographs failed to suggest the correct diagnosis in 32% of cases (44). Tuberculosis could not be diagnosed when radiographs appeared normal (13% of cases), showed minimal radiographic abnormalities, such as linear opacities or calcified granulomas, or showed atypical patterns of disease, such as diffuse opacities mimicking PCP. High-resolution CT scan may demonstrate parenchymal abnormalities in patients with normal radiographs and characteristic findings in patients with nonspecific radiographic findings. Hartman et al. (46) assessed the accuracy of CT scan interpretation in 102 patients with AIDS who had proven intrathoracic disease. On CT scan, mycobacterial infection was correctly suggested as the first choice of diagnosis in 44% of patients, and was among the top three choices in 77% of 26 patients who had tuberculosis or MAC infection.
Similar to the radiograph, high-resolution CT scan in patients with AIDS and tuberculosis shows a greater prevalence of mediastinal lymph node enlargement and miliary spread and lower prevalence of cavitation, endobronchial spread of infection and consolidation than in nonimmunocompromised patients (37,47,48).
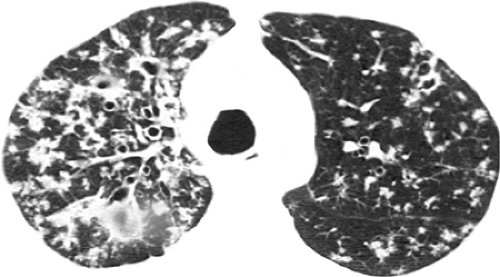
Leung et al. (47) compared the CT scan findings of 42 HIV-positive and 42 HIV-negative patients who had pulmonary tuberculosis. Findings seen with significantly lower frequency in HIV-positive patients compared to HIV-negative patients were cavitation (19% vs. 55%), consolidation (43% vs. 69%), and endobronchial spread (57% vs. 90%) resulting in centrilobular nodular opacities and “tree-in-bud” pattern (see Figs. 7.7 and 7.8) (49). Conversely, a miliary pattern was seen in 17% of HIV-positive patients and in none of the negative ones (see Fig. 7.9). Laissy et al. (37) compared the conventional and high-resolution CT scan findings in 29 HIV-positive and 47 HIV-negative patients who had newly diagnosed pulmonary tuberculosis. HIV-positive patients demonstrated significantly lower frequency of cavitation (24% vs. 49%). Cavitation was seen in only 13% of HIV-positive patients who had <200 CD4 T cells per mm3 compared to 50% of HIV-positive patients who had 200 or more CD4 T cells per mm3. On the other hand, lymphadenopathy was significantly more common in patients who had <200 CD4 T cells per mm3 (70% vs. 33%).
Mycobacterium avium-intracellulare Complex
MAC accounts for most nontuberculous mycobacterial infections seen in patients with AIDS (50,51,52). MAC infection usually results from primary exposure rather than reactivation of latent organisms. It tends to occur in the late stage of AIDS, when immune deficiency is severe and the CD4 lymphocyte count drops below 50 cells per mm3 (53).
Diagnosis of pulmonary nontuberculous mycobacterial infection is often difficult because isolation of the organism from sputum or bronchoalveolar lavage (BAL) fluid may be the result of airway colonization rather than infection (53). The diagnosis may be established from the combination of positive culture of sputum or BAL fluid, appropriate clinical and radiologic findings, and a therapeutic response. Approximately 20% of chest radiographs in patients with MAC-related pulmonary disease are normal (50). The most common findings include mediastinal or hilar lymphadenopathy. The pulmonary manifestations, when present, resemble those of tuberculosis and include multifocal patchy areas of consolidation or ill-defined nodules that may cavitate (see Fig. 7.10) (50,53,54). Pleural effusions are more common in MAC than in tuberculosis but miliary disease is rare (53).
Immune Reconstitution Inflammatory Syndrome
The radiographic and CT scan manifestations of tuberculosis in HIV-positive patients have changed considerably since the introduction of HAART (13). Because HAART results in partial restoration of cell-mediated immunity, patients with AIDS receiving HAART are more likely to show a pattern resembling postprimary tuberculosis. Busi Rizzi et al. (13) reviewed the chest radiographs in 209 HIV-infected patients with culture-confirmed pulmonary tuberculosis. CT images were also reviewed in 42 patients whose chest radiographs were normal or showed only questionable abnormalities. Postprimary pattern became more frequent after 1996 when HAART came into clinical use, being seen in 82% (27/33) of patients receiving HAART compared to 44% (77/176) of patients not on HAART (p <0.001). A primary pattern was significantly more frequent (p <0.001) in patients with more severe immunosuppression (CD4 lymphocyte <200 per mm3) (13).
Although HAART has resulted in a marked decrease in the frequency of opportunistic infections among HIV-infected individuals, some HAART-treated patients exhibit
paradoxical clinical deterioration, despite satisfactory control of viral replication and improvement in CD4 lymphocyte counts (55,56). This clinical deterioration, known as immune reconstitution inflammatory syndrome (IRIS) or immune restoration syndrome (57), is a result of an exuberant inflammatory response (55,56). In this condition, previously subclinical infections become clinically manifested or preexisting infections clinically deteriorate. IRIS is most frequently associated with mycobacterial infections and results in clinical deterioration of patients with tuberculosis and MAC infection (55,56,58). Treatment includes continuation of therapy for tuberculosis or MAC, continuation of effective HAART, and use of anti-inflammatory agents. Although the clinical manifestations of IRIS are sometimes dramatic, and result in substantial morbidity, most patients improve with therapy (55,56,59).
paradoxical clinical deterioration, despite satisfactory control of viral replication and improvement in CD4 lymphocyte counts (55,56). This clinical deterioration, known as immune reconstitution inflammatory syndrome (IRIS) or immune restoration syndrome (57), is a result of an exuberant inflammatory response (55,56). In this condition, previously subclinical infections become clinically manifested or preexisting infections clinically deteriorate. IRIS is most frequently associated with mycobacterial infections and results in clinical deterioration of patients with tuberculosis and MAC infection (55,56,58). Treatment includes continuation of therapy for tuberculosis or MAC, continuation of effective HAART, and use of anti-inflammatory agents. Although the clinical manifestations of IRIS are sometimes dramatic, and result in substantial morbidity, most patients improve with therapy (55,56,59).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree