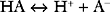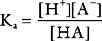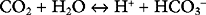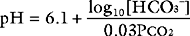Chapter 31 Acid-Base Disturbances
In this chapter, the principles of normal acid-base homeostasis are briefly reviewed and the common disturbances encountered in cardiac surgery patients are discussed. In a short summary such as this, many details are touched on only briefly or are oversimplified. In particular, the Stewart principle1 for analysis of acid-base disturbance is mentioned only in passing (Box 31-1).
BOX 31-1 The Stewart Physiochemical Approach to Acid-Base Analysis
The approach to acid-base analysis described by Stewart1 is based on the fundamental principles of physical chemistry and describes the mechanism of acid-base disturbances. These principles are as follows:
From these principles, one can derive the three independent variables influencing pH:
The main implication of the Stewart approach is that the concentration of hydrogen ions or bicarbonate cannot change unless one of the three independent variables changes. Similarly, changes caused by adding or removing hydrogen ions or bicarbonate in a compartment are accommodated by changes in the dissociation of water unless one or more of the independent variables changes. Acid-base disorders can therefore be classified in relation to the derangements of the independent variables. Metabolic acidoses are caused by a reduced SID or by an increased Atot, whereas metabolic alkaloses are caused by an increased SID or a reduced Atot. However, because no mechanisms appear to control Atot, changes in Atot are not considered to be acid-base disorders by some authors.12
ACID-BASE PHYSIOLOGY
Normal Regulation of pH
Buffers
Buffering ability is maximal when the concentration of the weak acid and its conjugate base are equal; that is, when [HA] = [A−], which occurs when the hydrogen ion concentration of the solution is equal to the dissociation constant (Ka) of the buffer; put another way, when the pH of the solution is equal to the pKa of the buffer (where pKa = the −log10Ka). To function effectively, a buffer must have a pKa of within 1.0 unit of the pH of the solution being buffered.
Buffering Systems Within the Body.
The bicarbonate buffer system is as follows:
Despite not having the same form as Equation 33-1, the bicarbonate buffer system reacts to changes in pH in a similar manner: the addition of H+ shifts the equation to the left, generating CO2 and H2O; the removal of H+ shifts the equation to the right, generating HCO3− (and H+). The pKa of the bicarbonate buffer system is 6.1, which at first glance seems too far removed from the pH of plasma to be very effective. However, because the concentration of bicarbonate in extracellular fluid is high (24 mmol/l) and because the bicarbonate buffer system is open, with the ability to independently control carbon dioxide and bicarbonate concentrations, it is quantitatively the most important buffer of the extracellular fluid.
The histidine amino acid found on hemoglobin and plasma proteins (pKa 6.5) constitutes the majority of the nonbicarbonate buffering in the extracellular fluid. Although hemoglobin is an intracellular protein, the permeability of the red cell membrane is such that it can be considered an extracellular buffer.
Role of the Lungs: Control of Paco2
The partial pressure of dissolved carbon dioxide in arterial blood (Paco2) is inversely proportional to alveolar ventilation and directly proportional to the rate of production of carbon dioxide (see Eq. 1-17). The carbon dioxide partial pressure in arterial blood is tightly controlled to a value close to 5.2 κPa (40 mmHg), corresponding to a concentration of dissolved gas of 1.2 mmol/l. A reduction in alveolar ventilation leads to an increase in Paco2, which drives the equilibrium of Equation 31-3 to the right, causing a fall in pH. This condition is termed respiratory acidosis. Conversely, an increase in alveolar ventilation drives the Paco2 down and the equilibrium of Equation 31-3 to the left, causing an increase in pH. This condition is termed respiratory alkalosis.
Role of the Kidneys: Control of Plasma HCO3− Concentration
On a normal diet, about 80 mmol/day of nonvolatile (non-carbon-dioxide or metabolic) acid is generated each day, mainly by the metabolism of proteins. This acid is buffered in the plasma by the bicarbonate buffer system and excreted as carbon dioxide gas, resulting in a net loss of bicarbonate. The cells of the distal convoluted tubule of the kidney regenerate this lost bicarbonate. In the process of regenerating bicarbonate, the distal tubular cells secrete an equimolar amount of hydrogen ions into the tubular fluid, resulting in acidification of the urine. With normal renal function, approximately 400 to 500 mmol of bicarbonate can be regenerated in this way (or, put another way, the kidneys can clear 400 to 500 mmol of H+ per day, providing that urinary buffering is ideal),2,3 resulting in a urinary pH as low as 4.5. In this way, the kidneys regulate the plasma bicarbonate concentration (normal range 24 to 28 mmol/l) in the face of a changing metabolic acid load. The larger the metabolic acid load, the lower the urinary pH. This renal mechanism takes some hours to days to be fully effective.
From Equation 31-3 it is seen that an excess of hydrogen ions leads to a fall in plasma bicarbonate concentration, a condition known as metabolic acidosis. Conversely, the loss of hydrogen ions results in an increase in plasma bicarbonate concentration, a condition known as a metabolic alkalosis.
Assessment of Acid-Base Status
Three parameters are used to assess acid-base status: pH, Paco2, and HCO3−. Both pH and Paco2 are measured directly by the blood-gas analyzer, whereas bicarbonate concentration is calculated (automatically) from the Henderson-Hasselbalch equation. Because bicarbonate concentration is influenced by changes in the partial pressure of carbon dioxide (due to shifts in the equilibrium shown in Equation 31-3), blood gas analyzers also report the standard bicarbonate or the base excess. The standard bicarbonate is what the actual bicarbonate concentration would be if the blood gas sample were equilibrated with carbon dioxide at 5.2 κPa in fully oxygenated blood at 37°C. The base excess is the amount (in mmol/l) of acid that would be required to titrate the blood sample to a pH of 7.4 at a Paco2 of 5.2 κPa at 37°C. A negative base excess indicates that there is excess acid in the blood; that is, a metabolic acidosis exists. Abnormalities of the standard bicarbonate and base excess provide a measure of the metabolic component of an acid-base disorder.