1.4 Acid–base balance
The basics
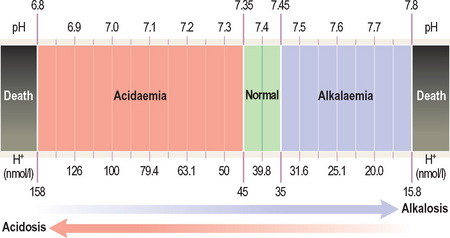
An acidosis is any process that lowers blood pH whereas an alkalosis is any process that raises blood pH.
It is a negative logarithmic scale (Figure 10). The ‘negative’ means that pH values get lower as the H+ concentration increases (so a pH of 7.1 is more acidic than 7.2). The ‘logarithmic’ means that a shift in pH by one number represents a 10-fold change in H+ concentration (so 7 is 10 times more acidic than 8).
Maintaining acid–base balance
What generates H+ ions in our bodies?
Metabolism of protein produces hydrochloric, sulphuric and other so-called ‘metabolic acids’.
H+ ions must, therefore, be removed to maintain normal blood pH.
What removes H+ ions from our bodies?
Renal (metabolic) mechanisms
The kidneys are responsible for excreting metabolic acids. They secrete H+ ions into urine and reabsorb HCO3 from urine. HCO3 is a base (and therefore accepts H+ ions), so it reduces the concentration of H+ ions in blood. The kidneys can adjust urinary H+ and HCO3 excretion in response to changes in metabolic acid production.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree