Chapter 7
Access for Hemodialysis
How frail is man, for despite his wisdom and strength, it is a mere few drops of water that spare his life. More so, how frail is the hemodialysis-dependent patient for whom the basic essentials of life are not enough. For this patient, vascular access is as important for survival as water. As physicians caring for these patients, the onus to provide and maintain this type of access has fallen squarely on our shoulders. Since the 1940s, we have acknowledged our responsibility toward these patients and have explored various vascular access methods.1–11
Today, more than half a century later, despite all our efforts, a method for obtaining reliable and completely trouble-free, high-flow vascular access still eludes us. This does not mean that progress has not been made over the past 60 years. To the contrary, modern vascular access methods, such as surgically created native and prosthetic arteriovenous fistulas (AVFs), are a vast improvement over earlier solutions and have gained universal acceptance in the dialysis community. Unfortunately, although these methods are close to ideal, they have many failings.
Native AVFs take a long time to mature or, in many patients, never mature.12–15 Prosthetic AVFs mature rapidly but fail frequently.16,17 Additionally, neither method provides immediate access for hemodialysis. Only intravenous catheters can offer this attribute.
Intravenous catheters are readily available, can be inserted easily, and can be used immediately. As such, these catheters have gained remarkable popularity. In 1993, 37% of newly diagnosed end-stage renal disease (ESRD) patients were receiving hemodialysis using a central venous catheter 1 month after initiation of their treatment.18 Additionally, it is estimated that 30% of hemodialysis-dependent patients have central venous catheters as their permanent type of vascular access.19 Unfortunately, the “ideal” features of central venous catheters are offset by the high morbidity associated with these devices relative to that of other types of access.20 Malfunction, infection, and central venous stenoses are frequent occurrences.21–27 For these reasons, the National Kidney Foundation Dialysis Outcomes Quality Initiative (NKF-DOQI) Work Group recommends that fewer than 10% of chronic dialysis patients be maintained on long-term catheter-based hemodialysis.28
As meritorious as the DOQI recommendation is, widespread attainment of this goal in the United States is unlikely to occur in the near future. The dialysis population is aging, and more of these patients have severe cardiac and peripheral vascular disease limiting their access options. Additionally, the current well-entrenched practice pattern of delaying the establishment of vascular access until the last possible moment continues to increase the clinician’s dependence on central venous catheters for both short- and long-term access solutions. Given this state of affairs, it is essential that clinicians placing these catheters must understand how to maximize the function of these devices while minimizing adverse consequences, which not only cause immediate patient morbidity but also often destroy central veins and ultimately prevent establishing durable arteriovenous (AV) accesses. Thus, this chapter discusses the different types of dialysis catheters and the indication for their use, and it highlights the techniques for proper implantation. Additionally, immediate and long-term outcomes as well as the cost-effectiveness of image-guided placement are reviewed.
HEMODIALYSIS CATHETERS
Catheter Design
To accomplish hemodialysis, blood must be removed from the body and passed through a system containing a semipermeable membrane to remove toxic metabolites and unwanted fluid. Once cleansed, the flowing blood is returned to the systemic circulation. Thus, for continuous hemodialysis, a circuit must be established with two separate access channels moving blood in opposite directions. To avoid recirculating the same treated blood, each of these channels must be located away from the other. This concept of separated dual-channel circulatory access is the basis of every hemodialysis catheter design. The challenge has been to translate this concept into a device that maximizes dual-channel flow rates and minimizes recirculation.
Recirculation has been the easiest challenge to overcome. Catheter channels do not need to be separated by great distances to reduce recirculation to an acceptably low level. If the draw channel is upstream to the return channel, a separation of only 2 to 3 cm is all that is needed.29,30 Unfortunately, establishing and maintaining high flow rates have presented a much greater challenge. On average, 120 L of blood must be processed at each hemodialysis session. For this to occur in a reasonable time frame, blood flow rates of 350 to 500 mL per minute are required. Attaining flow rates of this magnitude through a central venous catheter requires that the channels possess a suitably large diameter. Additionally, the wall of at least one of the channels must be rigid enough to withstand a draw pressure of 150 to 250 mm Hg without collapsing. Because flow rates are predominantly dependent on the inner diameter of each channel (Poiseuille law), an increase in this diameter is the primary way to achieve higher flow rates. The most direct manner in which this can be accomplished is to increase the overall diameter of the catheter. Unfortunately, the morbidity associated with central venous catheters increases as the outer diameter is enlarged. Thus, in designing these devices, a balance between catheter diameter and optimal flow rates must be achieved to maximize the risk-tobenefit ratio.
Current adult hemodialysis catheters range in size from approximately 10 to 14.5 French (F).31–33 Many of these catheters are designed as a single tube with two separate channels. Other designs rely on a two-catheter system, which consists of two separate single-channel catheters that are placed independently.31 All these devices are constructed of polyurethane or silicone rubber. Polyurethane, in comparison to silicone rubber, possesses a high tensile strength.34 This property allows for the construction of catheters with thinner walls that can withstand high flow rates. A thinner wall translates into a larger ratio of the inner diameter to the outer diameter, which in turn means higher sustainable flow rates for a given outer diameter. Unfortunately, polyurethane is susceptible to kinking with acute angulations. Additionally, polyurethane undergoes an enzymatic degradation process known as environmental stress cracking.34,35 This process, albeit quite slow, can weaken the catheter and result in catheter fragmentation and embolization. Silicone rubber, in contrast to polyurethane, is extremely biostable. It is soft and very flexible, which reduces the likelihood of endothelial injury and catheter kinks. Silicone rubber, however, has a low tensile strength, necessitating a smaller ratio of the inner diameter to the outer diameter compared with polyurethane. In today’s environment, this feature is problematic. Higher flow rates have been the focus of most new dialysis catheter designs, and manufacturers have all but abandoned the use of silicone rubber in favor of polyurethane materials.
Catheter Types
Central venous catheters for hemodialysis are designed for either short- or long-term use. Short-term, or acute, catheters, as opposed to long-term catheters, do not have a Dacron retention cuff and are not tunneled. They are designed specifically for rapid over-the-wire placement. For this type of placement to be possible, the catheter shaft must be rigid so that it can be advanced through the subcutaneous tissues. For the same reason, the catheter tip also must be tapered. Thus, once access is established into a central vein, the hemodialysis catheter can be advanced over the wire into position. The advantage of this type of catheter is in its ease of placement, which enables these catheters to be inserted at the patient’s bedside with minimal difficulty. Additionally, the rigid catheter can withstand high negative aspiration pressures, permitting adequate flow rates with a smaller catheter diameter. A smaller catheter diameter obviously contributes to the ease of implantation.
With implantation ease at the core of this catheter design, it is no surprise that a vast majority of these catheters are placed at the bedside, through the internal jugular, subclavian, or femoral vein without image guidance. These catheters are available in a straight or curved shaft configuration (Fig. 7–1). The straight shaft catheter is ideal for subclavian and femoral placement. The extension limbs of a straight shaft catheter, however, when placed in the internal jugular vein (IJV), often assume an awkward and uncomfortable position just below the patient’s ear. The curved shaft design displaces the extension limbs of the catheter downward, away from the patient’s neck onto the chest wall. This configuration is far more comfortable for the patient when the IJV is used for access. Another design modification involves curving the extension limbs back on themselves instead of the catheter shaft (Fig. 7–2). This design, similar to the curved shaft design, displaces the extension limbs downward into a more comfortable position.
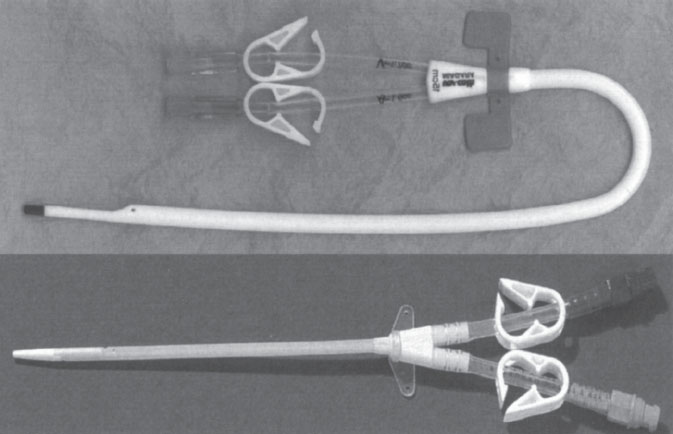
Figure 7–1 Straight (Mahurkar Catheter; Tyco Health Care, Kendall, Mansfield, MA, U.S.A.) and curved acute hemodialysis catheters (Niagra Bard Access Systems, Salt Lake City, UT, U.S.A.)
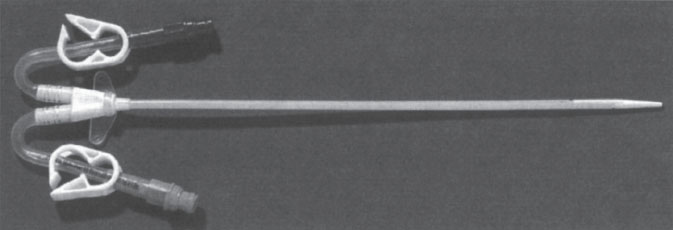
Figure 7–2 Mahurkar curved extension catheter (Tyco Health Care, Kendall, Mansfield, MA, U.S.A.).
Chronic Catheters
Unfortunately, the characteristics of the acute hemodialysis catheter, which include a rigid catheter shaft and a tapered tip, do not make this type of catheter suitable for longterm use.36 Conceptually, it is easy to see how a stiff, pointed catheter could cause significant injury to the superior vena cava (SVC) or right atrium (RA) if left in place a long time.3 What constitutes a long period never has been precisely defined; however, it is generally accepted that this type of catheter should not remain implanted for more than 2 to 3 weeks when access is through the subclavian or IJV.38 When the femoral vein is accessed, this type of catheter should not be left in place longer than 5 days.39 Therefore, for long-term catheter access, a different catheter design is required.
Long-term or chronic catheters are designed to be soft so that endovascular trauma can be minimized. These catheters are constructed from soft polyurethane or silicone rubber and therefore have little column strength. Consequently, these catheters do not possess the rigidity necessary to permit over-the-wire placement through subcutaneous tissues. To place this type of catheter into the central venous system, a pathway must be created through which the soft catheter shaft can be advanced. This can be accomplished through the use of a peel-away sheath consisting of a rigid dilator supporting a thin, circular tube designed to split lengthwise into two. The sheath-dilator combination is rigid enough to be advanced through subcutaneous tissues over a wire. With the sheath in the central vein, the rigid dilator is removed, leaving only the thin-walled tube, which is of sufficient diameter to allow passage of the soft access catheter through it into the central venous system. Once the access catheter is placed, the sheath is split and torn away, leaving only the access catheter in place.
The flexibility and softness of the catheter shaft are not the only distinction between the acute and chronic hemodialysis access catheter. Chronic hemodialysis catheters are also tunneled and possess a polyester retention cuff. Tunneling helps to reduce the likelihood of infection and enables the catheter’s exit site to be positioned away from the venotomy to a more convenient location.40 The polyester cuff promotes tissue ingrowth, which prevents the catheter from dislodging and helps to create a barrier against infection.41 These features enable this type of catheter to remain within the central venous system for a prolonged period.42 Currently, there is no maximum implantation time recommendation for these catheters. If needed, this type of catheter can be left in place indefinitely; however, vascular injury and vessel occlusion are the inevitable consequences of having any type of catheter in the central venous circulation for a prolonged period. Therefore, a strenuous effort should be made to reduce the overall length of time a central venous dialysis catheter remains in place. In the hemodialysis-dependent patient, this can be accomplished by hastening the creation of more permanent arterial-venous access solutions. With this mindset, chronic hemodialysis catheters, as a bridge to more permanent access, should rarely remain in place for longer than 3 months.
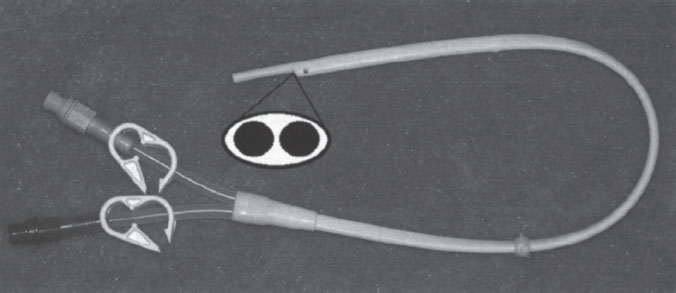
Figure 7–3 Quinton Permacath catheter (Tyco Health Care, Kendall, Mansfield, MA, U.S.A.).
Three months can be a very long time when a chronic access catheter fails to perform adequately. Ideally, this type of catheter should deliver a flow rate of at least 300 mL per minute.43 Unfortunately, achieving and maintaining this goal have been problematic. In addressing this problem, many different chronic catheter configurations designed to improve function have been marketed to implanting physicians. These catheter designs can be divided into three broad groups: singlecatheter design, dual-catheter design, and a composite design.
The single-catheter design consists of a single tube with two separate channels. The shape of the catheter and the configuration of channels within it are what individualize each of the access catheters in this group. An oval-shaped catheter with two round channels is one of the earliest catheter designs (Fig. 7–3). This design has a large outer diameter relative to the diameter of each channel. For this reason, catheters of this design are no longer popular. The most popular single-catheter configuration is the “double D.” This design takes a round catheter and places a septum down the center. This septum divides the catheter into two D-shaped channels (Fig. 7–4). This configuration uses available space very efficiently, and the overall inner diameter of each of the channels is large in relation to outer diameter of the catheter. A similar catheter configuration to the “double D” is the Circle C (Horizon Medical Products, Manchester, GA, U.S.A.). This catheter is a circular tube that has an asymmetrically positioned smaller circular tube within it (Fig. 7–5). A configuration of this type has a draw channel that is larger than the infusion channel, permitting a higher flow rate through the more functionally vulnerable of the two channels.
The dual-catheter design involves placing two single-lumen catheters through independent venous access points. Typically, these two points of access are adjacent to each other in the same vein; however, this need not be the case. Each individual catheter can be placed through a different central vein with the distal ends positioned in the RA. Each of the catheter ends must be positioned so that recirculation is avoided. This is accomplished by positioning the aspiration cannula 3 to 5 cm upstream from the infusion cannula. The Tesio catheter (Medical Components, Inc., Harleysville, PA, U.S.A.) and the SchonCath (AngioDynamics, Queensbury, NY, U.S.A.) are two commercially available catheters that utilize this design.
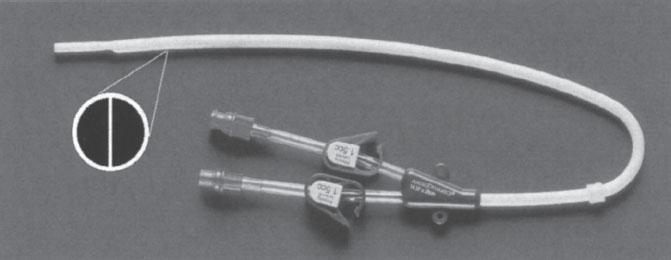
Figure 7–4 More-Flow catheter (AngioDynamics, Inc., Queensbury, NY, U.S.A.).
The Tesio catheter consists of two 10-F single-lumen catheters that are inserted through a central vein and tunneled independently onto the chest wall (Fig. 7–6). Each of these catheters is held in place by a large polyester cuff. This design enables high flow rates with reduced diameter venotomies. Reducing the caliber of each venotomy may reduce implantation complications. Additionally, two independent, free-floating cannulas may help to eliminate catheter occlusion. The extra time required to implant this device and the overall presence of more catheter material in the central vein, potentially causing central vein occlusion, are clear disadvantages.
The SchonCath is two 9-F single-lumen catheters that are conjoined for a short segment at their midportion (Fig. 7–7). Just beyond this junction, each of the two catheter segments is inserted into a central vein. The attachment point then is buried in the subcutaneous tissue at the venous entry site, and the back ends of each catheter are tunneled to a site on the chest wall. Hence, the attachment point between the two catheters, referred to by the manufacturer as the Hemolock anchoring hub, holds the entire catheter in place without using polyester cuffs. This device possesses the same theoretic advantages of the Tesio catheter, but it does not rely on tissue ingrowth into a cuff to prevent dislodgment. Disadvantages are analogous to the Tesio catheter, with the added difficulty of having to perform a more extensive dissection at the venotomy site to remove or replace the catheter.
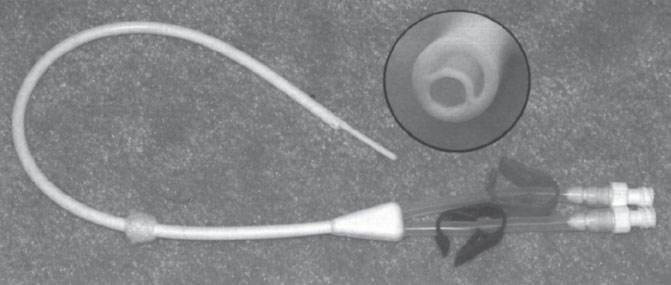
Figure 7–5 Circle C catheter (Horizon Medical Products, Manchester, GA, U.S.A.).
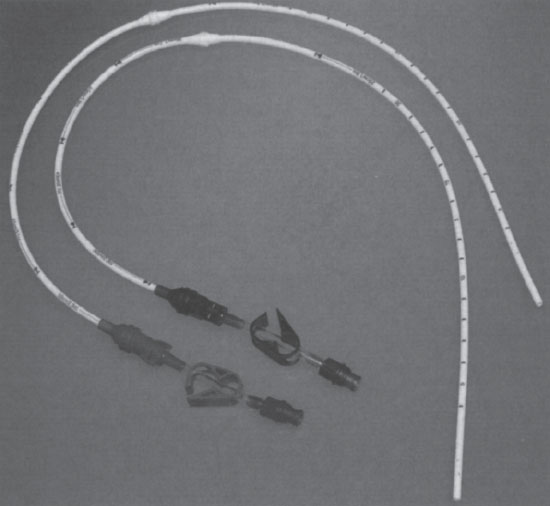
Figure 7–6 Tesio catheter (Medical Components, Inc., Harleysville, PA, U.S.A.).
A recent catheter design collages the advantages of the dual- and single-catheter systems. This composite design is easy to implant as a single-catheter device yet has two independent free-floating cannulas. This patented design, known as the Ash-Split Catheter (Medical Components, Inc.) has two D-shaped cannulas joined together to form a round catheter (Fig. 7–8). The joint holding these two cannulas together is designed to give way easily so that each of the two lumens can be split apart just before the device is placed in the central vein. Splitting the catheter gives rise to two separate free-floating lumens in the same vessel, which, similar to the dual catheter design, theoretically improves flow and increases long-term patency. Unfortunately, no data are currently available to support or refute this contention.
HEMODIALYSIS PORTS
Externalized catheters, such as those previously described, are easy to place and provide immediate high-flow access for hemodialysis. Unfortunately, these devices malfunction frequently and are susceptible to infection. In an effort to reduce the complications associated with these catheters, manufacturers have explored the idea of attaching these catheters to a subcutaneous reservoir. The concept of totally implanted venous access devices is not new. Totally implanted infusion devices have been available since the early 1980s; however, the high flow requirement of hemodialysis and the need of repetitive access present significant technical challenges.
The most significant challenge for these devices is that of repetitive transcutaneous large-bore cannula access. The device, which is a gateway to the central circulation, must open wide once a cannula is inserted and then rapidly close tightly when it is removed. Additionally, with frequent access, the device must be extremely resistant to bacterial contamination and the tissue and skin around the device must remain intact. Other important issues that must be addressed are ease of device access, functional longevity, and ease of repair. Currently, two devices address these issues in different ways: the Dialock Port, manufactured by Biolink Corp. (Middleboro, MA, U.S.A.) and the LifeSite, manufactured by Vasca, Inc. (Tewksbury, MA, U.S.A.).
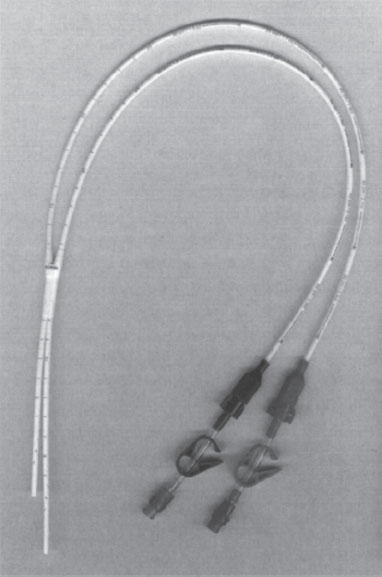
Figure 7–7 SchonCath (AngioDynamics, Queensbury, NY, U.S.A.).
The Dialock Port is a single titanium rectangular container that has two needle-accessible passageways (Fig. 7–9). Each passageway incorporates a normally closed valve assembly. The valves are opened only by insertion of the specifically designed Dialock needles. The device is accessed along its side in such a way that the cannulas are in line with the access catheters. The access catheters themselves are designed of thin-walled silicone reinforced with a metal braid. The catheters normally are implanted into the IVJ by separate IJV punctures. The device then is placed subcutaneously on the chest wall. Currently, this device is available overseas but is not yet approved by the U.S. Food and Drug Administration (FDA) for sale in the United States.
The LifeSite device is a stainless steel port containing a single valve. The valve is connected to a 12 F silicone catheter, which is inserted into the central venous circulation (Fig. 7–10). For hemodialysis, the standard approach involves two valves implanted adjacent to one another, with both cannulas inserted into the IJV. Alternatively, the valves could be implanted at separate locations, with each cannula entering a different central vein. The device is accessed with standard 14-gauge fistula needles inserted perpendicularly into the device. When the needle enters the device channel, the valve is opened, allowing direct communication with the venous cannula (Fig. 7–11).
In the initial clinical trial, the valve entry channel was cleansed with Chlorpactin, a mild bleach solution, to prevent device contamination and infection. Subsequent investigation demonstrated 70% isopropyl alcohol to be a better cleansing solution that substantially reduces the infection rate for this device.44 Cleansing is accomplished by insertion of a 25-gauge needle into the valve channel and the injection of 1 mL of the alcohol solution. The 25-gauge needle does not actuate the valve mechanism, and no communication with the central venous system is established during the alcohol wash. This device had been under clinical investigation in the United States since June 1997 and was approved for sale in the United States by the FDA in August 2000.
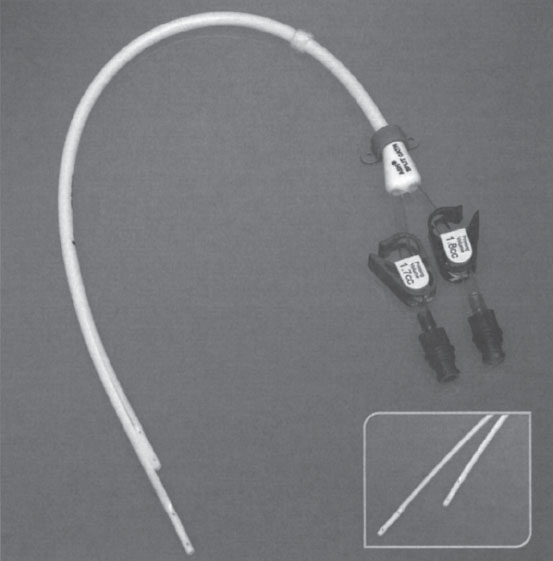
Figure 7–8 Ash-Split catheter (Medical Components, Inc., Harleysville, PA, U.S.A.).
Catheter Selection
In the past, hemodialysis access catheter diversity was limited, and as a consequence catheter selection was simple. With one or two less-than-ideal catheters of each type, acute or chronic, the implanting physician chose the one with which he or she was most comfortable. For the better, in the past decade, the number of available access catheters has increased dramatically. Currently, several manufacturers produce hemodialysis access catheters, each unique and yet similar in design. In many instances, only subtle differences, such as the number of side holes at the catheter tip, distinguish one catheter from another. In this era of abundance, it has become increasingly difficult to determine which catheter is best. Given these difficulties, it is important to keep in mind the criteria used to select a hemodialysis catheter. These criteria, in order of priority, include: function, risk, ease of insertion, maintenance ease, patient comfort, and cost.
Catheter function is without question the most important criterion to consider when selecting a suitable catheter. Adequate function, defined by the DOQI as a sustained blood flow rate of greater than 300 mL per minute, was difficult to achieve with many of the older, small-diameter catheters. Recently, larger-diameter (i.e., more than 14.0 F) polyurethane devices have been introduced that can sustain flow rates greater than 400 mL per minute.33 In most adults, these newer catheters should be chosen over smaller-diameter, lower-flow devices. Not every hemodialysis patient requires catheters with very high flow rates, however. Some patients cannot tolerate high-flux hemodialysis and are dialyzed at a rate that is well below the capacity of these large-bore devices. Additionally, the recommended hemodialysis flow rate for pediatric patients is only 5 mL per kilogram per minute, and catheter flow capacities above 250 mL per minute are unnecessary. In this small group of patients, the potential risks associated with larger-diameter, high-flow catheters can be avoided by using smaller-diameter 8 F and 13.5 F catheters.
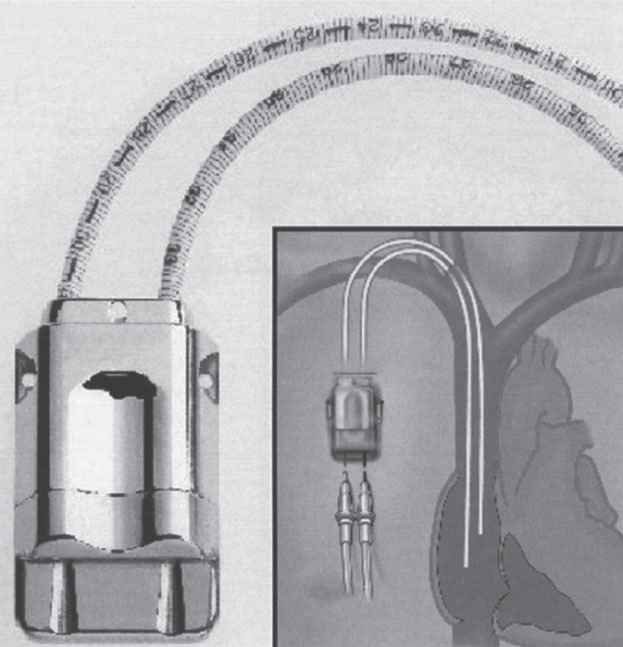
Figure 7–9 Dialock port (Biolink Corp., Middleboro, MA, U.S.A.).
Effort always should be made to minimize the risks of placing a chronic hemodialysis catheter. It is therefore imperative that the device selected for implantation help in this goal. For most devices, the overall risk is similar. Some devices, however, by virtue of their design, are inherently more risky than others. Large-bore devices inserted through large-bore peel-away sheaths are intuitively more likely than smaller-diameter catheters to result in an air embolus. Additionally, the large-bore Ash-Split catheter, because of the separation between the two lumens, is likely at greater risk for air embolus. Other devices possess different risks, some of which include excessive thrombogenicity and material instability. Material instability, usually the consequence of a manufacturing flaw, can result in device fracture and embolization (Fig. 7–12). The implanting physicians must be familiar with the unique risks of each device and weigh these risks against any potential benefits when selecting a suitable hemodialysis access catheter.
Ease of insertion and maintenance are other factors that should be considered when selecting a hemodialysis access device. Single-catheter, dual-lumen devices are easier to insert than dual-catheter devices, which require the vein to be accessed twice, the placement of two peel-away sheaths, and the creation of two separate subcutaneous tunnels. Additionally, dual-catheter systems are more difficult to maintain. When a dual-catheter system malfunctions, often both catheters need to be exchanged. This requires the placement of two guidewires, the dissection of two retention cuffs, and, if appropriate, disruption of two fibrin sleeves. Even removing dual-catheter systems is more time consuming than single-catheter systems simply because two retention cuffs, as opposed to one, must be dissected free.
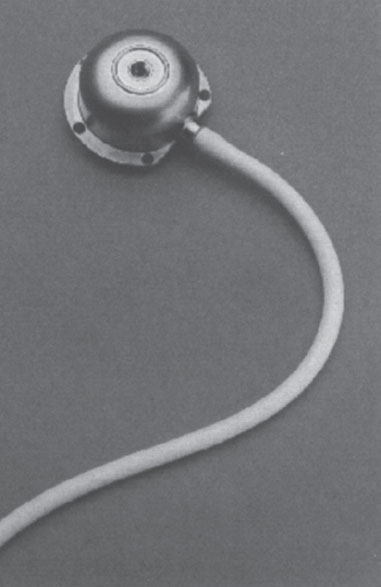
Figure 7–10 LifeSite (Vasca, Inc., Tewksbury, MA, U.S.A.).

Figure 7–11 The 14-gauge needle displaces two ball bearings laterally as it passes through the opening of the device. These bearings force the access cup downward, releasing the pinch on the valve at the base, opening a channel to blood flow.
Cost is always an important consideration when selecting a hemodialysis access device. In most instances, the cost difference between two devices is negligible, allowing outcome parameters to carry greater weight in the selection decision. The cost difference between a tunneled and a totally implanted device is dramatic, however, and therefore must be taken into consideration. The cost of a totally implanted hemodialysis device is approximately $3,600.00, which, on average, is more than 12 times that of a tunneled catheter. To justify this initial cost, the totally implanted device, over time, must reduce the overall cost of catheter maintenance by minimizing risk and maximizing function and device longevity. Whereas early data suggest that this is true, this cost-to-benefit ratio can be obtained in a patient who will require veno-venous hemodialysis for several months or years. Thus, proper patient selection is critical. Ultimately, further studies will be required to confirm the cost savings that these devices can afford.
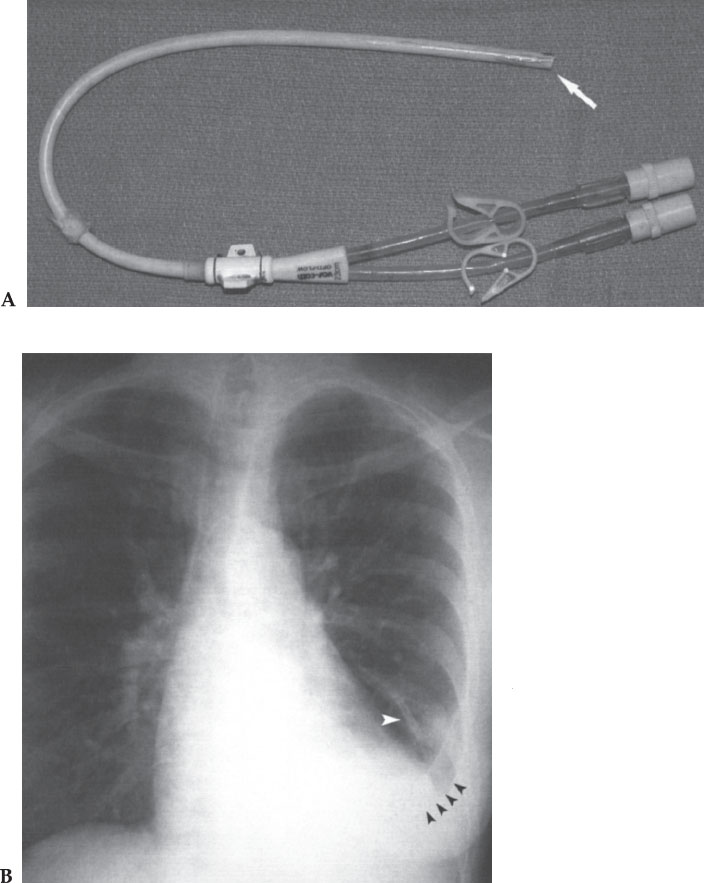
Figure 7–12 A chronic hemodialysis access catheter was placed in this patient 3 months earlier. Removal of the catheter was required for persistent fever. (A) When the catheter was removed, it was noted that the tip had separated from the catheter (arrow). (B) The tip embolized to the lung and lodged in a pulmonary artery branch (white arrowhead) resulting in a chronic infection and pleural effusion (black arrowheads). The tip could not be removed percutaneously, and the patient eventually required a lobectomy to resolve the infection.
Only a handful of investigators have compared different types of catheters in randomized, controlled trials. Trerotola and colleagues compared the 14.5 F Ash-Split catheter with the 13.5 F Hickman hemodialysis catheter and found both catheters able to provide acceptable flow rates.33 Not surprisingly, the Ash-Split catheter, having a larger luminal diameter, was capable of higher flow rates than the smaller 13.5 F Hickman catheter. This higher flow capability can enhance dialysis efficiency and reduce the incidence of catheter failure. The tradeoff, as noted in this study, is a slightly longer insertion time and higher complication rate. More recently, Richard and co-workers evaluated hemodialysis catheters of similar luminal diameter.45 The 14.5 F Ash-Split catheter (Medcomp, Harleysville, PA, U.S.A.), the 14.5 F Opti-flow catheter (Bard Access Systems, Salt Lake City, UT, U.S.A.), and the 10 F Tesio catheter (Medcomp) were investigated in this comparative trial. The investigators concluded that the Opti-flow and Ash-Split catheters were faster and easier to place than the Tesio catheter; however, no significant difference in function or catheter longevity was found. The risk of catheter placement was minimally higher for the Tesio catheter, but this difference was not statistically significant.
Few, if any, additional studies exist that prospectively compare the functionality of hemodialysis catheters. Furthermore, new hemodialysis catheters, which have never been evaluated, are constantly being introduced into the marketplace. The manufacturers promote each of these devices as being superior to all others. Unfortunately, in most instances, there is no evidence to support these claims. Given the dearth of data on this topic, it is nearly impossible to make recommendations on which specific catheter to select.
HEMODIALYSIS CATHETER IMPLANTATION
Site Selection
The most important aspect of placing a central venous catheter for hemodialysis is access site selection. Commonly, the subclavian and the jugular veins are used to introduce these large-bore catheters into the central circulation. Traditionally, the subclavian veins have been the most frequently used point of access because of their convenient chest wall location and the clinician’s broad familiarity with subclavian access techniques.41,46,51 Unfortunately, for many reasons, the incidence of complications associated with subclavian vein access is high.52,53 Complications such as pneumothorax and central venous thrombosis are more likely to occur with subclavian vein access, even when imaging techniques are used.54,55 These complications, which are infrequent, are almost always symptomatic. However, subclavian vein stenosis, which is normally asymptomatic, is a significant problem for the hemodialysis-dependent patient because of its devastating effect on future long-term access options and the frequency with which it occurs. Barrett and colleagues reported a 50% incidence of subclavian vein stenosis in patients with acute subclavian access catheters.26 They also noted that the incidence of stenosis was related to the duration of catheterization. Others reported similar findings.53,56–61 In our practice, we also found this to be true, and we believe that the incidence of subclavian vein stenosis approaches 100% if the access catheter is left in place for a long enough period. Therefore, every effort should be made to avoid subclavian vein cannulation in any patient who is a candidate for upper-extremity AV access.51 So critical is this point that this practice restriction should not be limited to large-bore dialysis catheters; rather, it should be applied to any type of infusion catheter in this patient population. Additionally, the patient population subjected to this restriction should include not only patients with chronic renal failure but also those with borderline renal function who someday might develop renal insufficiency.
HELPFUL HINT
The only scenario in which subclavian vein access would be considered appropriate is for the patient who has exhausted all surgical access options in the extremity ipsilateral to the desired site of access. In all other situations, the jugular vein is the preferred point of access.
Internal jugular vein access should be considered the access site of choice when placing hemodialysis access catheters. This access, over the short term, reduces the risk of pneumothorax and over the long term is less likely to cause problematic central vein stenoses.58–61 Yet IJV access is still avoided occasionally when placing central venous access devices for hemodialysis. The explanation for this practice is likely attributable to the acute angulation that the catheter sometimes must make as it comes down the neck to its exit site on the chest wall. This angulation often results in a catheter kink that compromises luminal diameter and hence reduces blood flow rates (Fig. 7–13). In our early experience, this was a significant problem. Our initial solution was to modify the course of our subcutaneous tunnel tract. This, unfortunately, resulted in odd catheter exit sites that were poorly tolerated by patients. So uncomfortable was this solution that patients would obstinately refuse jugular vein access. In searching for an alternative solution to this problem, we developed the low posterior approach to the IJV. This technique, described in detail later in this chapter, permits the catheter to assume a gentle, kink-free curve as it courses through its subcutaneous tunnel to a comfortable, unobtrusive exit site on the chest wall (Fig. 7–14). As a consequence of this new approach, catheter function improved, and patient satisfaction increased. The disadvantages once commonly associated with IJV access were eliminated, allowing us to completely abandon subclavian vein access with just one caveat: The jugular veins had to be traversable with a guidewire.
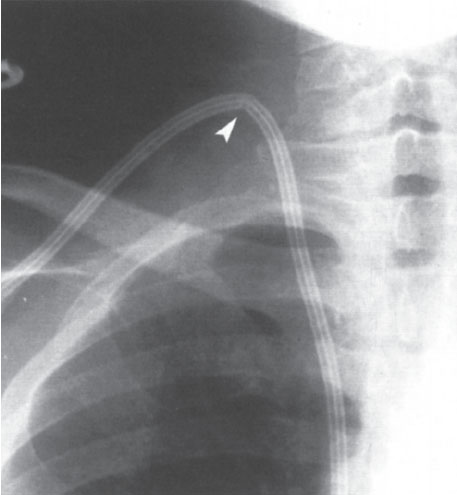
Figure 7–13 The anterior or middle approach to the jugular vein forces the soft-access catheter to assume an acute angle as it is tunneled into the chest wall



