Bradyarrhythmias
There are two broad categories of abnormally slow heart rhythms—the failure of pacemaker cells to generate appropriate electrical impulses (disorders of automaticity) and the failure to propagate electrical impulses appropriately (heart block).
Failure of Impulse Generation
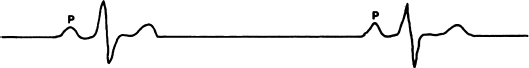
Failure of SA nodal automaticity, resulting in an insufficient number of electrical impulses emanating from the SA node (i.e. sinus bradycardia (Figure 2.1)), is the most common cause of bradyarrhythmias. If the slowed heart rate is insufficient to meet the body’s demands, symptoms result. Symptomatic sinus bradycardia is called sick sinus syndrome. If sinus slowing is profound, subsidiary pacemakers located near the AV junction can take over the pacemaker function of the heart. The electrophysiology study, as we will see in Chapter 5, can be useful in assessing SA nodal automaticity.
Failure of Impulse Propagation
The second major cause of bradyarrhythmias is the failure of the electrical impulses generated by the SA node (or by subsidiary atrial pacemakers) to conduct normally to the ventricles. This condition, known as heart block or AV block, implies an abnormality of conduction velocity and/or refractoriness in the conducting system. Because conduction of the electrical impulse to the ventricles depends on the function of the AV node and the His–Purkinje system, heart block is virtually always due to AV nodal or His–Purkinje disease.
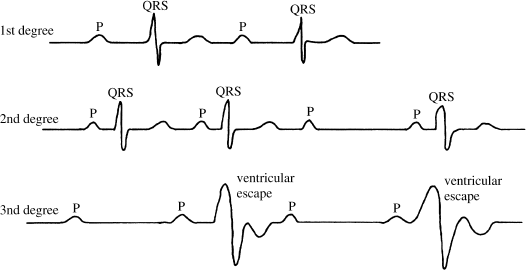
Heart block is classified into three categories based on severity (Figure 2.2). First-degree AV block means that, while all atrial impulses are transmitted to the ventricles, intraatrial conduction, conduction through the AV node, and/or conduction through the His bundle is slow (manifested on the ECG by a prolonged PR interval). Second-degree AV block means that conduction to the ventricles is intermittent; that is, some impulses are conducted and some are blocked. Third-degree AV block means that block is complete and no atrial impulses are conducted to the ventricles.
Figure 2.2 Three categories of heart block. In first-degree block (top tracing), all atrial impulses are conducted to the ventricles, but conduction is slow (the PR interval is prolonged). In second-degree block (middle tracing), some atrial impulses are conducted and some are not. In third-degree block (bottom tracing), none of the atrial impulses are conducted to the ventricles.
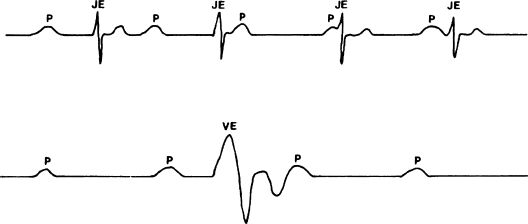
If third-degree AV block is present then sustaining life depends on the function of subsidiary pacemakers distal to the site of block. The competence of these subsidiary pacemakers, and therefore the patient’s prognosis, depends largely on the site of block (Figure 2.3). When block is within the AV node, subsidiary pacemakers at the AV junction usually take over the pacemaker function of the heart, resulting in a relatively stable, non-life-threatening heart rhythm, with a rate often in excess of 50 beats/min. On the other hand, if block is distal to the AV node, the subsidiary pacemakers tend to produce a profoundly slow (usually less than 40 beats/min) and unstable heart rhythm.
Figure 2.3 Examples of escape pacemakers. When block is localized to the AV node (top tracing), junctional escape pacemakers (JE) are usually stable enough to prevent hemodynamic collapse. When block is located in the distal conducting tissues (bottom tracing), escape pacemakers are usually located in the ventricles (VE) and are slower and much less stable.
If heart block is less than complete (i.e. first- or second-degree), it is still important to pinpoint the site of block to either the AV node or the His–Purkinje system. First- or second-degree block in the AV node is benign and tends to be nonprogressive. Thus, implanting a permanent pacemaker is rarely required. First- and especially second-degree block distal to the AV node, on the other hand, tends to progress to a higher degree of block; prophylactic pacing is often indicated.
Differentiating the site of heart block requires careful evaluation. This evaluation can usually be made noninvasively by studying the surface ECG and taking advantage of the fact the AV node has rich autonomic innervation and the His–Purkinje system does not. Sometimes, however, the electrophysiology study is useful in locating the site of block. Chapter 5 considers heart block in detail.
Tachyarrhythmias
Cardiac tachyarrhythmias can cause significant mortality and morbidity. It is the ability of the electrophysiology study to address the evaluation and treatment of tachyarrhythmias that has brought this procedure into widespread use. We will discuss three mechanisms for tachyarrhythmias—automaticity, reentry, and triggered activity.
Automaticity
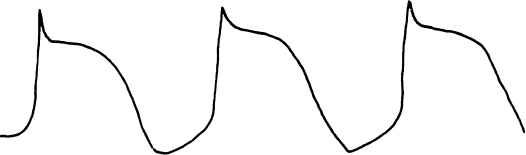
Automaticity has been discussed as a normal pacemaker function of the heart. When abnormal acceleration of phase 4 activity occurs in some location of the heart, an automatic tachyarrhythmia is said to occur (Figure 2.4). Such an abnormal automatic focus can appear in the atria, the AV junction, or the ventricles (thus leading to automatic atrial, junctional, or ventricular tachycardia).
Figure 2.4 Abnormal automaticity causes the rapid generation of action potentials and thus inappropriate tachycardia.
Automaticity is not a common cause of tachyarrhythmias, probably accounting for less than 10% of all abnormal tachyarrhythmias. Automatic tachyarrhythmias are usually recognizable by their characteristics and the settings in which they occur.
In gaining an understanding of the automatic tachyarrhythmias, it is helpful to consider the characteristics of sinus tachycardia, which is a normal automatic tachycardia. Sinus tachycardia usually occurs as a result of appropriately increased sympathetic tone (for instance, in response to increased metabolic needs during exercise). When sinus tachycardia develops, the heart rate gradually increases from the basic (resting) sinus rate; when sinus tachycardia subsides, the rate likewise decreases gradually.
Similarly, automatic tachyarrhythmias often display a warm-up and warm-down in rate when the arrhythmia begins and ends. Analogous to sinus tachycardia, automatic tachyarrhythmias also often have metabolic causes, such as acute cardiac ischemia, hypoxemia, hypokalemia, hypomagnesemia, acid–base disorders, high sympathetic tone, and the use of sympathomimetic agents. Therefore, automatic arrhythmias are often seen in acutely ill patients, often in the intensive-care setting, with all the attendant metabolic abnormalities. For example, acute pulmonary disease can lead to multifocal atrial tachycardia, the most common type of automatic atrial tachycardia. Induction of, and recovery from, general anesthesia can cause surges in sympathetic tone, and automatic arrhythmias (both atrial and ventricular) can result. In addition, acute myocardial infarction is often accompanied by early ventricular arrhythmias that are most likely automatic in mechanism.
Of all tachyarrhythmias, automatic arrhythmias most closely resemble an “itch of the heart,” and it is tempting to apply the salve of antiarrhythmic drugs. Antiarrhythmic drugs can sometimes decrease automaticity; automatic arrhythmias, however, should be treated primarily by identifying and reversing the underlying metabolic cause.
Automatic tachyarrhythmias cannot be induced by programmed pacing techniques, so these arrhythmias are generally not amenable to provocative study in the electrophysiology laboratory.
Reentry
Reentry is the most common mechanism for tachyarrhythmias; it is also the most important, because reentrant arrhythmias cause the deaths of hundreds of thousands of people every year. Fortunately, reentrant arrhythmias lend themselves nicely to study in the electrophysiology laboratory. It was the recognition that most tachyarrhythmias are reentrant in mechanism and that the electrophysiology study can help significantly in assessing reentrant arrhythmias that led to widespread proliferation of electrophysiology laboratories from the early 1980s onward.
Unfortunately, the mechanism of reentry is not simple to explain or to understand, and the prerequisites for reentry seem on the surface to be unlikely at best. The failure to understand (and possibly to believe in) reentry has helped keep the electrophysiology study an enigma to most people in the medical profession. The following explanation of reentry therefore errs on the side of simplicity and might offend some electrophysiologists. If the reader can keep an open mind and accept this explanation for now, we hope to show later (in Chapters 6 and 7) that reentry is a compelling explanation for most cardiac tachyarrhythmias.
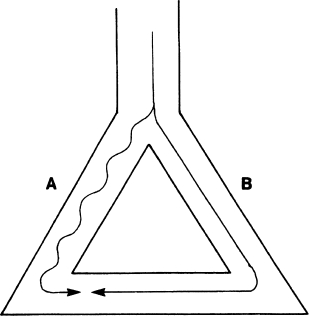
Reentry requires that the following criteria be met (Figure 2.5). First, two roughly parallel conducting pathways (shown as pathways A and B) must be connected proximally and distally by conducting tissue, thus forming a potential electrical circuit. Second, one of the pathways (pathway B in our example) must have a refractory period that is substantially longer than that of the other pathway. Third, the pathway with the shorter refractory period (pathway A) must conduct electrical impulses more slowly than the other pathway.
Figure 2.5 Prerequisites for reentry. An anatomic circuit must be present in which two portions of the circuit (pathways A and B in the figure) have electrophysiologic properties that differ from one another in a critical way. In this example, pathway A conducts electrical impulses more slowly than pathway B, while pathway B has a longer refractory period than pathway A.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree