Ablation of Autonomic Ganglia
Hiroshi Nakagawa
Katsuaki Yokoyama
Benjamin Scherlag
Vikram Katari
Hiroshi Aoyama
Sara Foresti
Warren Jackman
Several different approaches have been developed for catheter ablation of atrial fibrillation (AF) as described in previous chapters. The similar success rates (70%-85%) among these different procedures (with or without complete isolation of the pulmonary veins, with or without left atrial linear lesions, with or without complete block across the linear lesions (1, 2, 3, 4, 5, 6, 7, 8, 9, 10) and ablation at sites of complex fractionated atrial electrograms during AF [11]) suggests that there are additional factors important to the success of the ablation procedure besides pulmonary vein (PV) isolation and continuous linear lesions. A likely candidate for a factor common to all these procedures is ablation of some of the major atrial autonomic ganglionated plexi (GP).
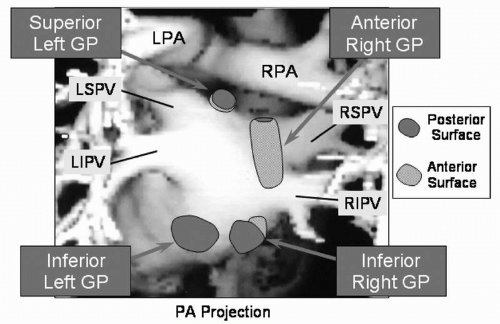
GP (containing afferent neurons from the atrial myocardium and central autonomic nervous system, efferent cholinergic and adrenergic neurons, and interconnecting neurons) are present on the epicardial surface of the atria. There are at least seven major atrial GP (12). The four major GP associated with the left atrium are located superior and medial to the left superior PV ostium (superior left GP), 2 to 4 cm inferior to the left inferior PV ostium (inferior left GP), anterior to the right superior and inferior PV ostia (anterior right GP), and 1 to 3 cm inferior to the right inferior PV ostium (inferior right GP, Fig. 14.1) (13, 14, 15, 16). Other GP are located at the medial aspect of the SVC-right atrial junction (SVC-RA GP), in the crux of the heart between the right and left atria, close to the coronary sinus ostium and the inferior vena cava (Crux GP), and within the ligament of Marshall.
The initiation and maintenance of AF is significantly enhanced by simultaneous parasympathetic stimulation (shortening the refractory period of the atrial and PV myocardium) and sympathetic stimulation (calcium loading resulting in triggered firing from PV myocardium) (17). Recent experimental study has shown in dogs that electrical high-frequency stimulation (HFS) of epicardial GP markedly shortens atrial refractory periods at sites close to the GP and allows initiation of sustained AF by a single atrial extrastimulus (single premature atrial contraction) delivered close to the
stimulated GP (Fig. 14.2, 14.3 and 14.4) (13,16). HFS in the left atrium away from the stimulated GP showed no decrease in atrial refractory period or enhanced inducibility of AF. Pharmacological stimulation of the GP (injection of acetylcholine in the epicardial fat pad) initiates AF with rapid firing from the adjacent PV (Fig. 14.5) (18).
stimulated GP (Fig. 14.2, 14.3 and 14.4) (13,16). HFS in the left atrium away from the stimulated GP showed no decrease in atrial refractory period or enhanced inducibility of AF. Pharmacological stimulation of the GP (injection of acetylcholine in the epicardial fat pad) initiates AF with rapid firing from the adjacent PV (Fig. 14.5) (18).
We found the GP could be localized from the endocardium by HFS (13, 14, 15, 16). When close to a GP, endocardial HFS produced a vagal response identified by a marked lengthening of the R-R interval during AF (Fig. 14.3A). Electrograms recorded from the endocardium close to the GP during AF exhibited a change in pattern to complex fractionated atrial electrograms (CFAE) during epicardial HFS of that GP (13,16,18). These potentials are identical to the CFAE described at successful ablation sites by Nademanee (11). Endocardial ablation at those sites eliminated the vagal response by HFS and decreases CFAE in that area and in the adjacent PV (Fig. 14.3B) (13,16,19).
All of the current approaches to AF ablation include radiofrequency (RF) applications to some of the GP area. For example, the sites of CFAE used as the ablation target by Nademanee et al. surround the GP (11,20). Similarly, the sites of vagal response during RF applications described by Pappone et al. (21) as indicating greater ablation success are close to some of the GP, often the superior left GP.
In this chapter, we describe the technique of GP ablation as an adjunctive to PV antrum isolation in patients with paroxysmal and persistent AF.
We obtain magnetic resonance angiography 1 to 3 days prior to study to identify left atrial and PV anatomy. Electrophysiologic study is performed under general anesthesia using propofol or desflurane with vecuronium for the paralytic agent. We found that cisatracurium (Nimbex) can block GP function and inhibit induction of sustained AF (22). Double transseptal puncture is performed under intracardiac echocardiographic guidance (ICE). PV angiography (during adenosine-induced AV block) and ICE are used to facilitate electroanatomic mapping.
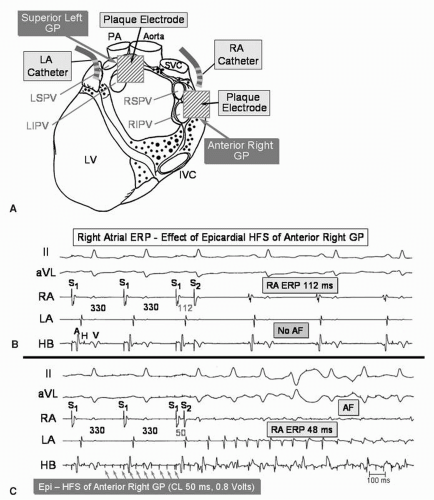 Figure 14.2. The effect of epicardial high-frequency stimulation (HFS) of the epicardial fat pad containing the anterior right GP (ARGP) on the effective refractory period (and inducibility of AF) of the right atrium close to the ARGP, before and after endocardial ablation of the GP in a canine model. A: After thoracotomy, plaque electrodes are placed over the fat pads containing the anterior right GP and the superior left GP. Catheter electrodes are sutured to the right atrium and left atrium, approximately 5 mm from the plaque electrodes Heart Rhythm. 2007;14:S61.) B: In the absence of HFS of the ARGP, the right atrial effective refractory period is 112 ms. Atrial fibrillation (AF) is not induced by a single extrastimulus from the right atrium. C: Continuous HFS (cycle length 50 ms, 0.8 volts without atrial capture) applied from the epicardial plaque electrodes on the ARGP fat pad, resulting in a significant shortening of the atrial refractory period to 48 ms. Note the extrastimulus at a coupling interval of 50 ms captures the atrium and induces AF. D: Fluoroscopic image in the left anterior oblique (LAO) projection showing the location of epicardial and endocardial electrodes. After placement of the epicardial electrodes, the chest was closed and transseptal puncture was performed under fluoroscopic guidance. A 20-pole Lasso catheter was positioned just outside the right superior pulmonary vein (RSPV). A saline-irrigated ablation catheter was positioned on the right atrial endocardium very close to the ARGP (ABL). Other catheters include an epicardial electrode catheter sutured on the right atrium close to the plaque electrode on the ARGP (EPi RA), an epicardial electrode catheter sutured on the left atrium close to the plaque electrode on the SLGP (EPi LA), and His bundle catheter located in the noncoronary aortic cusp (HB-Ao). E, F: After endocardial ablation of the ARGP, a single atrial extrastimulus is delivered to the right atrium at couple intervals of 130 ms and 128 ms during continuous epicardial HFS of the fat pad containing the ARGP. The right atrial refractory period close to the ARGP during continuous epicardial HFS prolonged to 128 ms and the atrial extrastimulus at 130 ms failed to initiate AF. This indicates that endocardial ablation successfully eliminated the effects of epicardial HFS of the GP and that baseline GP activation shortens local atrial refractoriness.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|